Randomized Phase III Study of Alisertib or Investigator's Choice (Selected Single Agent) in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma
- PMID: 30707661
- PMCID: PMC6494247
- DOI: 10.1200/JCO.18.00899
Randomized Phase III Study of Alisertib or Investigator's Choice (Selected Single Agent) in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma
Abstract
Purpose: The aim of this open-label, first-in-setting, randomized phase III trial was to evaluate the efficacy of alisertib, an investigational Aurora A kinase inhibitor, in patients with relapsed/refractory peripheral T-cell lymphoma (PTCL).
Patients and methods: Adult patients with relapsed/refractory PTCL-one or more prior therapy-were randomly assigned 1:1 to receive oral alisertib 50 mg two times per day (days 1 to 7; 21-day cycle) or investigator-selected single-agent comparator, including intravenous pralatrexate 30 mg/m2 (once per week for 6 weeks; 7-week cycle), or intravenous gemcitabine 1,000 mg/m2 or intravenous romidepsin 14 mg/m2 (days 1, 8, and 15; 28-day cycle). Tumor tissue (disease subtype) and imaging were assessed by independent central review. Primary outcomes were overall response rate and progression-free survival (PFS). Two interim analyses and one final analysis were planned.
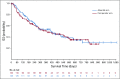
Results: Between May 2012 and October 2014, 271 patients were randomly assigned (alisertib, n = 138; comparator, n = 133). Enrollment was stopped early on the recommendation of the independent data monitoring committee as a result of the low probability of alisertib achieving PFS superiority with full enrollment. Centrally assessed overall response rate was 33% for alisertib and 45% for the comparator arm (odds ratio, 0.60; 95% CI, 0.33 to 1.08). Median PFS was 115 days for alisertib and 104 days for the comparator arm (hazard ratio, 0.87; 95% CI, 0.637 to 1.178). The most common adverse events were anemia (53% of alisertib-treated patients v 34% of comparator-treated patients) and neutropenia (47% v 31%, respectively). A lower percentage of patients who received alisertib (9%) compared with the comparator (14%) experienced events that led to study drug discontinuation. Of 26 on-study deaths, five were considered treatment related (alisertib, n = 3 of 11; comparator, n = 2 of 15). Two-year overall survival was 35% for each arm.
Conclusion: In patients with relapsed/refractory PTCL, alisertib was not statistically significantly superior to the comparator arm.
Trial registration: ClinicalTrials.gov NCT01482962.
Figures

References
-
- Vose J, Armitage J, Weisenburger D, et al. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. - PubMed
-
- Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: Spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. - PubMed
-
- Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–636. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

