The role of PHOX2B-derived astrocytes in chemosensory control of breathing and sleep homeostasis
- PMID: 30707772
- PMCID: PMC6462490
- DOI: 10.1113/JP277082
The role of PHOX2B-derived astrocytes in chemosensory control of breathing and sleep homeostasis
Abstract
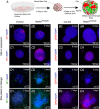
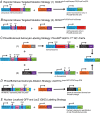
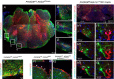
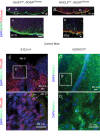
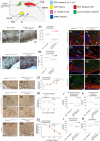
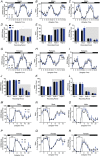
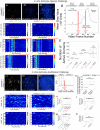
Key points: The embryonic PHOX2B-progenitor domain generates neuronal and glial cells which together are involved in chemosensory control of breathing and sleep homeostasis. Ablating PHOX2B-derived astrocytes significantly contributes to secondary hypoxic respiratory depression as well as abnormalities in sleep homeostasis. PHOX2B-derived astrocyte ablation results in axonal pathologies in the retrotrapezoid nucleus.
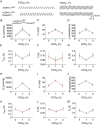
Abstract: We identify in mice a population of ∼800 retrotrapezoid nucleus (RTN) astrocytes derived from PHOX2B-positive, OLIG3-negative progenitor cells, that interact with PHOX2B-expressing RTN chemosensory neurons. PHOX2B-derived astrocyte ablation during early life results in adult-onset O2 chemoreflex deficiency. These animals also display changes in sleep homeostasis, including fragmented sleep and disturbances in delta power after sleep deprivation, all without observable changes in anxiety or social behaviours. Ultrastructural evaluation of the RTN demonstrates that PHOX2B-derived astrocyte ablation results in features characteristic of degenerative neuro-axonal dystrophy, including abnormally dilated axon terminals and increased amounts of synapses containing autophagic vacuoles/phagosomes. We conclude that PHOX2B-derived astrocytes are necessary for maintaining a functional O2 chemosensory reflex in the adult, modulate sleep homeostasis, and are key regulators of synaptic integrity in the RTN region, which is necessary for the chemosensory control of breathing. These data also highlight how defects in embryonic development may manifest as neurodegenerative pathology in an adult.
Keywords: Chemosensation; PHOX2B; Respiration; Sleep Homeostasis.
© 2019 The Authors. The Journal of Physiology © 2019 The Physiological Society.
Figures




References
-
- Amiel J, Laudier B, Attie‐Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C & Lyonnet S (2003). Polyalanine expansion and frameshift mutations of the paired‐like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33, 459–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

