The contribution of the locus coeruleus-norepinephrine system in the emergence of defeat-induced inflammatory priming
- PMID: 30707932
- PMCID: PMC6591045
- DOI: 10.1016/j.bbi.2019.01.021
The contribution of the locus coeruleus-norepinephrine system in the emergence of defeat-induced inflammatory priming
Abstract
Exposure to psychosocial stress is known to precipitate the emergence of stress related psychiatric disorders such as depression and anxiety. While mechanisms by which this occurs remain largely unclear, recent evidence points towards a causative role for inflammation. Neurotransmitters, such as norepinephrine (NE), are capable of regulating expression of proinflammatory cytokines and thus may contribute to the emergence of stress-related disorders. The locus coeruleus (LC) is the major source of norepinephrine (NE) to the brain and therefore the current study utilized N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4), an LC selective noradrenergic neurotoxin, to determine the discrete involvement of the LC-NE system in social defeat-induced inflammation in LC projection regions including the central amygdala (CeA), dorsal raphe (DR) and plasma. In the current study, rats were exposed to brief social defeat or control manipulations on 5 consecutive days. To determine whether a history of social defeat enhanced or "primed" the inflammatory response to a subsequent defeat exposure, all rats regardless of stress history were exposed to an acute social defeat challenge immediately preceeding tissue collection. As anticipated, prior history of social defeat primed inflammatory responses in the plasma and CeA while neuroinflammation in the DR was markedly reduced. Notably, DSP-4 treatment suppressed stress-induced circulating inflammatory cytokines independent of prior stress history. In contrast, neuroinflammation in the CeA and DR were greatly augmented selectively in DSP-4 treated rats with a history of social defeat. Together these data highlight the dichotomous nature of NE in stress-induced inflammatory priming in the periphery and the brain and directly implicate the LC-NE system in these processes.
Keywords: Central amygdala; DSP-4; Dorsal raphe; Inflammation; Locus coeruleus; Neuroinflammatory priming; Norepinephrine; Peripheral inflammatory priming; Resident intruder paradigm; Social defeat.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
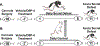

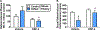



Comment in
-
The locus coeruleus may be a new target in regulating inflammation.Brain Behav Immun. 2019 Jul;79:18-19. doi: 10.1016/j.bbi.2019.04.018. Epub 2019 Apr 5. Brain Behav Immun. 2019. PMID: 30959176 No abstract available.
Similar articles
-
The protective effects of resveratrol on social stress-induced cytokine release and depressive-like behavior.Brain Behav Immun. 2017 Jan;59:147-157. doi: 10.1016/j.bbi.2016.08.019. Epub 2016 Aug 31. Brain Behav Immun. 2017. PMID: 27592314 Free PMC article.
-
Neurochemically distinct circuitry regulates locus coeruleus activity during female social stress depending on coping style.Brain Struct Funct. 2019 May;224(4):1429-1446. doi: 10.1007/s00429-019-01837-5. Epub 2019 Feb 14. Brain Struct Funct. 2019. PMID: 30767070 Free PMC article.
-
Inflammatory Factors Mediate Vulnerability to a Social Stress-Induced Depressive-like Phenotype in Passive Coping Rats.Biol Psychiatry. 2015 Jul 1;78(1):38-48. doi: 10.1016/j.biopsych.2014.10.026. Epub 2014 Nov 25. Biol Psychiatry. 2015. PMID: 25676490 Free PMC article.
-
Stress, antidepressant drugs, and the locus coeruleus.Brain Res Bull. 1994;35(5-6):545-56. doi: 10.1016/0361-9230(94)90168-6. Brain Res Bull. 1994. PMID: 7859112 Review.
-
Selective effects of DSP-4 on locus coeruleus axons: are there pharmacologically different types of noradrenergic axons in the central nervous system?Prog Brain Res. 1991;88:257-68. doi: 10.1016/s0079-6123(08)63815-7. Prog Brain Res. 1991. PMID: 1726027 Review.
Cited by
-
Norepinephrine May Exacerbate Septic Acute Kidney Injury: A Narrative Review.J Clin Med. 2023 Feb 9;12(4):1373. doi: 10.3390/jcm12041373. J Clin Med. 2023. PMID: 36835909 Free PMC article. Review.
-
Social adversity during juvenile age but not adulthood increases susceptibility to an immune challenge later in life.Neurobiol Stress. 2023 Feb 8;23:100526. doi: 10.1016/j.ynstr.2023.100526. eCollection 2023 Mar. Neurobiol Stress. 2023. PMID: 36844420 Free PMC article.
-
Individual differences in behavioral responses to predator odor predict subsequent stress reactivity in female rats.Stress. 2025 Dec;28(1):2479739. doi: 10.1080/10253890.2025.2479739. Epub 2025 Apr 3. Stress. 2025. PMID: 40181610
-
Voluntary wheel running as a promising strategy to promote autonomic resilience to social stress in females: Vagal tone lies at the heart of the matter.Auton Neurosci. 2024 Jun;253:103175. doi: 10.1016/j.autneu.2024.103175. Epub 2024 Apr 15. Auton Neurosci. 2024. PMID: 38677130 Free PMC article. Review.
-
Brain histamine and oleoylethanolamide restore behavioral deficits induced by chronic social defeat stress in mice.Neurobiol Stress. 2021 Mar 17;14:100317. doi: 10.1016/j.ynstr.2021.100317. eCollection 2021 May. Neurobiol Stress. 2021. PMID: 33869681 Free PMC article.
References
-
- Aghajanian GK, Wang RY, 1977. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res 122, 229–242. - PubMed
-
- Almeida DM, 2005. Resilience and Vulnerability to Daily Stressors Assessed via Diary Methods. Current Directions in Psychological Science 14, 64–68.
-
- Badowska-Szalewska E, Ludkiewicz B, Spodnik JH, Krawczyk R, Morys J, 2015. The influence of mild stressors on neurons containing interleukin-1beta in the central (CeA) and medial (MeA) amygdala in the ageing process of rats. Acta neurobiologiae experimentalis 75, 279–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

