A critical review of the role of M2PYK in the Warburg effect
- PMID: 30708038
- PMCID: PMC6525063
- DOI: 10.1016/j.bbcan.2019.01.004
A critical review of the role of M2PYK in the Warburg effect
Abstract
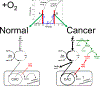
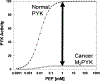
It is becoming generally accepted in recent literature that the Warburg effect in cancer depends on inhibition of M2PYK, the pyruvate kinase isozyme most commonly expressed in tumors. We remain skeptical. There continues to be a general lack of solid experimental evidence for the underlying idea that a bottle neck in aerobic glycolysis at the level of M2PYK results in an expanded pool of glycolytic intermediates (which are thought to serve as building blocks necessary for proliferation and growth of cancer cells). If a bottle neck at M2PYK exists, then the remarkable increase in lactate production by cancer cells is a paradox, particularly since a high percentage of the carbons of lactate originate from glucose. The finding that pyruvate kinase activity is invariantly increased rather than decreased in cancer undermines the logic of the M2PYK bottle neck, but is consistent with high lactate production. The "inactive" state of M2PYK in cancer is often described as a dimer (with reduced substrate affinity) that has dissociated from an active tetramer of M2PYK. Although M2PYK clearly dissociates easier than other isozymes of pyruvate kinase, it is not clear that dissociation of the tetramer occurs in vivo when ligands are present that promote tetramer formation. Furthermore, it is also not clear whether the dissociated dimer retains any activity at all. A number of non-canonical functions for M2PYK have been proposed, all of which can be challenged by the finding that not all cancer cell types are dependent on M2PYK expression. Additional in-depth studies of the Warburg effect and specifically of the possible regulatory role of M2PYK in the Warburg effect are needed.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures

References
-
- O’Connell K, Doran P, Gannon J, Ohlendieck K, Lectin-based proteomic profiling of aged skeletal muscle: decreased pyruvate kinase isozyme M1 exhibits drastically increased levels of N-glycosylation, Eur J Cell Biol, 87 (2008) 793–805. - PubMed
-
- Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, Hart GW, Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications, Mol Cell Proteomics, 1 (2002) 791–804. - PubMed
-
- Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J, Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer, Proteomics, 13 (2013) 2088–2099. - PubMed
-
- Chaiyawat P, Chokchaichamnankit D, Lirdprapamongkol K, Srisomsap C, Svasti J, Champattanachai V, Alteration of O-GlcNAcylation affects serine phosphorylation and regulates gene expression and activity of pyruvate kinase M2 in colorectal cancer cells, Oncol Rep, 34 (2015) 1933–1942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

