The Bispidinone Derivative 3,7-Bis-[2-(S)-amino-3-(1 H-indol-3-yl)-propionyl]-1,5-diphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one Dihydrochloride Induces an Apoptosis-Mediated Cytotoxic Effect on Pancreatic Cancer Cells In Vitro
- PMID: 30709047
- PMCID: PMC6384835
- DOI: 10.3390/molecules24030524
The Bispidinone Derivative 3,7-Bis-[2-(S)-amino-3-(1 H-indol-3-yl)-propionyl]-1,5-diphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one Dihydrochloride Induces an Apoptosis-Mediated Cytotoxic Effect on Pancreatic Cancer Cells In Vitro
Abstract
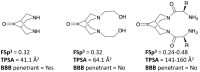
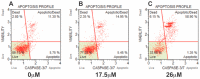
Pancreatic cancer (PC) is a complex, heterogeneous disease with a dismal prognosis. Current therapies have failed to improve survival outcomes, urging the need for discovery of novel targeted treatments. Bispidinone derivatives have yet to be investigated as cytotoxic agents against PC cells. The cytotoxic effect of four bispidinone derivatives (BisP1: 1,5-diphenyl-3,7-bis(2-hydroxyethyl)-3,7-diazabicyclo[3.3.1]nonan-9-one; BisP2: 3,7-bis-(2-(S)-amino-4-methylsulfanylbutyryl)-1,5-diphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one dihydrochloride; BisP3: [2-{7-[2-(S)-tert-butoxycarbonylamino-3-(1H-indol-3-yl)-propionyl]-9-oxo-1,5-diphenyl-3,7-diazabicyclo[3.3.1]non-3-yl}-1-(S)-(1H-indol-3-ylmethyl)-2-oxoethyl]-carbamic acid tertbutyl ester; BisP4: 3,7-bis-[2-(S)-amino-3-(1H-indol-3-yl)-propionyl]-1,5-diphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one dihydrochloride) was assessed against PC cell lines (MiaPaca-2, CFPAC-1 and BxPC-3). Cell viability was assessed using a Cell Counting Kit-8 (CCK-8) colorimetric assay, while apoptotic cell death was confirmed using fluorescence microscopy and flow cytometry. Initial viability screening revealed significant cytotoxic activity from BisP4 treatment (1 µM⁻100 µM) on all three cell lines, with IC50 values for MiaPaca-2, BxPC-3, and CFPAC-1 16.9 µM, 23.7 µM, and 36.3 µM, respectively. Cytotoxic treatment time-response (4 h, 24 h, and 48 h) revealed a 24 h treatment time was sufficient to produce a cytotoxic effect on all cell lines. Light microscopy evaluation (DAPI staining) of BisP4 treated MiaPaca-2 PC cells revealed dose-dependent characteristic apoptotic morphological changes. In addition, flow cytometry confirmed BisP4 induced apoptotic cell death induction of activated caspase-3/-7. The bispidinone derivative BisP4 induced an apoptosis-mediated cytotoxic effect on MiaPaca-2 cell lines and significant cytotoxicity on CFPAC-1 and BxPC-3 cell lines. Further investigations into the precise cellular mechanisms of action of this class of compounds are necessary for potential development into pre-clinical trials.
Keywords: Pancreatic cancer; apoptosis; bispidinone; cytotoxic; drug development; in vitro.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

