Proteases: History, discovery, and roles in health and disease
- PMID: 30710012
- PMCID: PMC6364759
- DOI: 10.1074/jbc.TM118.004156
Proteases: History, discovery, and roles in health and disease
Abstract
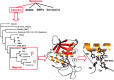
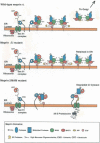
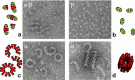
The Journal of Biological Chemistry (JBC) has been a major vehicle for disseminating and recording the discovery and characterization of proteolytic enzymes. The pace of discovery in the protease field accelerated during the 1971-2010 period that Dr. Herb Tabor served as the JBC's editor-in-chief. When he began his tenure, the fine structure and kinetics of only a few proteases were known; now thousands of proteases have been characterized, and over 600 genes for proteases have been identified in the human genome. In this review, besides reflecting on Dr. Tabor's invaluable contributions to the JBC and the American Society for Biochemistry and Molecular Biology (ASBMB), I endeavor to provide an overview of the extensive history of protease research, highlighting a few discoveries and roles of proteases in vivo In addition, metalloproteinases, particularly meprins of the astacin family, will be discussed with regard to structural characteristics, regulation, mechanisms of action, and roles in health and disease. Proteases and protein degradation play crucial roles in living systems, and I briefly address future directions in this highly diverse and thriving research area.
Keywords: astacins; meprins; metalloprotease; metalloproteinase; protein complex; protein degradation; protein domain; proteinase.
© 2019 Bond.
Conflict of interest statement
The author declares that she has no conflicts of interest with the contents of this article
Figures

References
-
- Levene P. A. (1905) The cleavage products of proteoses. J. Biol. Chem. 1, 45–58
-
- Robertson T. B. (1907) Note on the synthesis of a protein through the action of pepsin. J. Biol. Chem. 3, 95–99
-
- Robertson T. B. (1907) Studies in the chemistry of the ion-proteid compounds: IV. On some chemical properties of casein and their possible relation to the chemical behavior of other protein bodies, with especial reference to hydrolysis of casein by trypsin. J. Biol. Chem. 2, 317–383
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

