How myofilament strain and strain rate lead the dance of the cardiac cycle
- PMID: 30710504
- PMCID: PMC6589344
- DOI: 10.1016/j.abb.2019.01.034
How myofilament strain and strain rate lead the dance of the cardiac cycle
Abstract
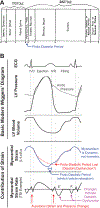
Movement of the myocardium can modify organ-level cardiac function and its molecular (crossbridge) mechanisms. This motion, which is defined by myocardial strain and strain rate (muscle shortening, lengthening, and the speed of these movements), occurs throughout the cardiac cycle, including during isovolumic periods. This review highlights how the left ventricular myocardium moves throughout the cardiac cycle, how muscle mechanics experiments provide insight into the regulation of forces used to move blood in and out of the left ventricle, and its impact on (and regulation by) crossbridge and sarcomere kinetics. We specifically highlight how muscle mechanics experiments explain how myocardial relaxation is accelerated by lengthening (strain rate) during late systole and isovolumic relaxation, a lengthening which has been measured in human hearts. Advancing and refining both in vivo measurement and ex vivo protocols with physiologic strain and strain rates could reveal important insights into molecular (crossbridge) kinetics. These advances could provide an improvement in both diagnosis and precise treatment of cardiac dysfunction.
Keywords: Diastole; Relaxation; Strain; Strain rate; Striated muscle; Systole.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


References
-
- Wiggers CJ, Am J Physiol 56 (1921) 415–438.
-
- Pombo JF, Troy BL, Russell RO Jr., Circulation 43 (1971) 480–490. - PubMed
-
- Folse R, Braunwald E, Circulation 25 (1962) 674–685. - PubMed
-
- Appleton CP, Firstenberg MS, Garcia MJ, Thomas JD, Cardiol Clin 18 (2000) 513–546, ix. - PubMed
-
- Redfield MM, Jacobsen SJ, Burnett JC Jr., Mahoney DW, Bailey KR, Rodeheffer RJ, Jama 289 (2003) 194–202. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

