Identification of β-synuclein on secretory granules in chromaffin cells and the effects of α- and β-synuclein on post-fusion BDNF discharge and fusion pore expansion
- PMID: 30711526
- PMCID: PMC6443492
- DOI: 10.1016/j.neulet.2019.01.056
Identification of β-synuclein on secretory granules in chromaffin cells and the effects of α- and β-synuclein on post-fusion BDNF discharge and fusion pore expansion
Abstract
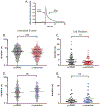
α-Synuclein is strongly implicated in the pathogenesis of Parkinson's disease as well as in other neurodegenerative diseases. However, its normal function in cells is not understood. The N-termini of α-, β-, and γ-synuclein contains six to seven 11-amino acid repeats that are predicted to form amphipathic helices. Membrane-binding and membrane-curving abilities of synuclein raise the possibility that synuclein could alter cellular processes that involve highly curved structures. In the present study we examined the localization of endogenous synuclein in bovine chromaffin cells by immunocytochemistry and its possible function to control protein discharge upon fusion of the granule with the plasma membrane by regulating the fusion pore. We found with quantitative immunocytochemistry that endogenous β-synuclein associates with secretory granules. Endogenous α-synuclein only rarely co-localizes with secretory granules. Overexpression of α-synuclein but not β-synuclein quickened the post- fusion discharge of BDNF-pHluorin by approxinately 30%. However, neither α- nor β-synuclein significantly altered curvature dynamics associated with fusion pore expansion that were measured by the combination of polarization and total internal reflection fluorescence microscopy (pTIRFM). Whatever the mechanism, the physiological significance of the small increased rate of post-fusion protein discharge caused by α-synuclein remains to be demonstrated, especially since endogenous β-, but not α-synuclein is the predominant synuclein isoform associated with chromaffin granules.
Keywords: Fusion pore; Immunocytochemistry; Secretion; Synuclein; TIRFM; pTIRFM.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures


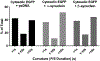
References
-
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D, {alpha}-Synuclein Overexpression in PC12 and Chromaffin Cells Impairs Catecholamine Release by Interfering with a Late Step in Exocytosis, Journal of Neuroscience 26 (2006) 11915–11922. - PMC - PubMed
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A, Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system, Neuron 25 (2000) 239–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

