Maternal titanium dioxide nanomaterial inhalation exposure compromises placental hemodynamics
- PMID: 30711534
- PMCID: PMC6422339
- DOI: 10.1016/j.taap.2019.01.024
Maternal titanium dioxide nanomaterial inhalation exposure compromises placental hemodynamics
Abstract
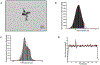
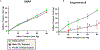
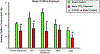
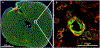
The fetal consequences of gestational engineered nanomaterial (ENM) exposure are unclear. The placenta is a barrier protecting the fetus and allowing transfer of substances from the maternal circulation. The purpose of this study was to determine the effects of maternal pulmonary titanium dioxide nanoparticle (nano-TiO2) exposure on the placenta and umbilical vascular reactivity. We hypothesized that pulmonary nano-TiO2 inhalation exposure increases placental vascular resistance and impairs umbilical vascular responsiveness. Pregnant Sprague-Dawley rats were exposed via whole-body inhalation to nano-TiO2 with an aerodynamic diameter of 188 ± 0.36 nm. On gestational day (GD) 11, rats began inhalation exposures (6 h/exposure). Daily lung deposition was 87.5 ± 2.7 μg. Animals were exposed for 6 days for a cumulative lung burden of 525 ± 16 μg. On GD 20, placentas, umbilical artery and vein were isolated, cannulated, and treated with acetylcholine (ACh), angiotensin II (ANGII), S-nitroso-N-acetyl-DL-penicillamine (SNAP), or calcium-free superfusate (Ca2+-free). Mean outflow pressure was measured in placental units. ACh increased outflow pressure to 53 ± 5 mmHg in sham-controls but only to 35 ± 4 mmHg in exposed subjects. ANGII decreased outflow pressure in placentas from exposed animals (17 ± 7 mmHg) compared to sham-controls (31 ± 6 mmHg). Ca2+-free superfusate yielded maximal outflow pressures in sham-control (63 ± 5 mmHg) and exposed (30 ± 10 mmHg) rats. Umbilical artery endothelium-dependent dilation was decreased in nano-TiO2 exposed fetuses (30 ± 9%) compared to sham-controls (58 ± 6%), but ANGII sensitivity was increased (-79 ± 20% vs -36 ± 10%). These results indicate that maternal gestational pulmonary nano-TiO2 exposure increases placental vascular resistance and impairs umbilical vascular reactivity.
Keywords: Engineered nanomaterials; Microcirculation; Placenta; Titanium dioxide nanoparticles.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


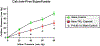


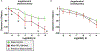
References
-
- Barker DJ 2007. The origins of the developmental origins theory. Journal of internal medicine, 261(5), 412–417. - PubMed
-
- Ema M, Hougaard KS, Kishimoto A, Honda K, 2016. Reproductive and developmental toxicity of carbon-based nanomaterials: A literature review. Nanotoxicology. 10(4),391–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

