Hematopoietic Cell Transplantation for Paroxysmal Nocturnal Hemoglobinuria in the Age of Eculizumab
- PMID: 30711779
- PMCID: PMC6615950
- DOI: 10.1016/j.bbmt.2019.01.033
Hematopoietic Cell Transplantation for Paroxysmal Nocturnal Hemoglobinuria in the Age of Eculizumab
Abstract
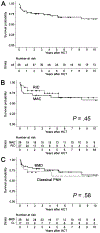
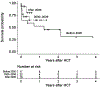
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired clonal hematopoietic cell disease characterized by the destruction of hematopoietic cells through activation of the complement system with manifestations that can be life-threatening including hemolysis, thrombosis, and marrow failure. Allogeneic hematopoietic cell transplantation (HCT) remains the sole cure for PNH, but eculizumab, a terminal complement inhibitor of C5, has been used to prevent complement-mediated hemolysis in patients with PNH since its approval by the Food and Drug Administration in 2007. We examined outcomes of HCT in patients with PNH to evaluate the effects of disease subtype, conditioning intensity, and eculizumab use either pre-HCT or post-HCT. Fifty-five patients with a diagnosis of PNH underwent at least 1 HCT, with 4 patients requiring a second HCT for graft failure. The median age at the time of first HCT was 30.0 years (range, 4.2 to 66.9 years). Seventeen patients (30.9%) had classical PNH, and the remaining 38 patients had PNH associated with another marrow disorder (aplastic anemia in 26 of the 38). Indications for HCT included pancytopenia in 47.3% of the patients, myeloid malignancy (myelodysplastic syndrome, myeloproliferative neoplasm, or acute myelogenous leukemia) in 21.8%, recurrent hemolysis in 20.0%, and thrombosis in 10.9%. Of the 55 first HCTs, 26 were performed with myeloablative conditioning, 27 were performed with reduced-intensity conditioning, and 2 sets of identical twins underwent HCT without any conditioning. Donor types included HLA-matched related in 38.2%, HLA-matched unrelated in 34.5%, single HLA-allele mismatched unrelated in 16.4%, umbilical cord blood in 5.5%, syngeneic in 3.6%, and HLA-haploidentical in 1.8%. The median duration of follow-up in surviving patients was 6.1 years (range, 2.1 to 46.1 years) after first HCT. The median time to neutrophil and platelet engraftment was 17 days and 19 days, respectively; all but 2 patients (96.3%) had sustained engraftment. Overall survival was 70% at 5 years. Neither the choice of conditioning intensity nor PNH subtype affected survival. Nineteen patients died during follow-up, including 12 patients before day +365. Six patients received treatment with eculizumab before HCT, and 2 were treated after HCT. All patients treated with eculizumab were alive at a median follow-up of 2.3 years (range, .2 to 6.9 years). Both patients treated with eculizumab after HCT had minimal to no acute GVHD (aGVHD), with grade I skin aGVHD in 1 patient and no aGVHD in the other patient, and no chronic GVHD at 2.1 and 4.1 years post-HCT, respectively. With the approval of eculizumab, the indications for HCT include persistent hemolysis, persistent thrombosis, and associated marrow failure. Administration of eculizumab before and after HCT warrants further study, particularly considering our observation of minimal to no GVHD in 2 patients who received eculizumab after HCT.
Keywords: Allogeneic transplantation; E eculizumab; PNH; Paroxysmal nocturnal hemoglobinuria.
Copyright © 2019 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: None
Figures
References
-
- Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. New England Journal of Medicine. 1995;333:1253–1258. - PubMed
-
- Hill A, Platts PJ, Smith A, et al. The incidence and prevalence of paroxysmal nocturnal hemoglobinuria (PNH) and survival of patients in Yorkshire (Abstract). Blood. 2006;108:985–985.
-
- Hugel B, Socie G, Vu T, et al. Elevated levels of circulating procoagulant microparticles in patients with paroxysmal nocturnal hemoglobinuria and aplastic anemia. Blood. 1999;93:3451–3456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

