Vasoactive Intestinal Polypeptide-Expressing Interneurons in the Hippocampus Support Goal-Oriented Spatial Learning
- PMID: 30713030
- PMCID: PMC6428605
- DOI: 10.1016/j.neuron.2019.01.009
Vasoactive Intestinal Polypeptide-Expressing Interneurons in the Hippocampus Support Goal-Oriented Spatial Learning
Abstract
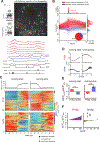
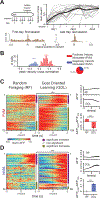
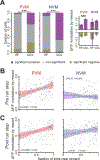
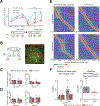
Diverse computations in the neocortex are aided by specialized GABAergic interneurons (INs), which selectively target other INs. However, much less is known about how these canonical disinhibitory circuit motifs contribute to network operations supporting spatial navigation and learning in the hippocampus. Using chronic two-photon calcium imaging in mice performing random foraging or goal-oriented learning tasks, we found that vasoactive intestinal polypeptide-expressing (VIP+), disinhibitory INs in hippocampal area CA1 form functional subpopulations defined by their modulation by behavioral states and task demands. Optogenetic manipulations of VIP+ INs and computational modeling further showed that VIP+ disinhibition is necessary for goal-directed learning and related reorganization of hippocampal pyramidal cell population dynamics. Our results demonstrate that disinhibitory circuits in the hippocampus play an active role in supporting spatial learning. VIDEO ABSTRACT.
Keywords: VIP; disinhibition; hippocampus; inhibition; place cell; reward; spatial learning; two-photon.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors have no competing interests related to this work.
Figures



Comment in
-
Disinhibition Goes Spatial.Neuron. 2019 Mar 20;101(6):994-996. doi: 10.1016/j.neuron.2019.03.006. Neuron. 2019. PMID: 30897364
References
-
- Acsady L, Arabadzisz D, and Freund TF (1996a). Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience 73, 299–315. - PubMed
-
- Acsady L, Gorcs TJ, and Freund TF (1996b). Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience 73, 317–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

