Multiplexed In Situ Imaging Mass Cytometry Analysis of the Human Endocrine Pancreas and Immune System in Type 1 Diabetes
- PMID: 30713110
- PMCID: PMC6436557
- DOI: 10.1016/j.cmet.2019.01.003
Multiplexed In Situ Imaging Mass Cytometry Analysis of the Human Endocrine Pancreas and Immune System in Type 1 Diabetes
Abstract
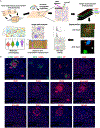
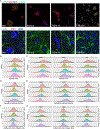
The interaction between the immune system and endocrine cells in the pancreas is crucial for the initiation and progression of type 1 diabetes (T1D). Imaging mass cytometry (IMC) enables multiplexed assessment of the abundance and localization of more than 30 proteins on the same tissue section at 1-μm resolution. Herein, we have developed a panel of 33 antibodies that allows for the quantification of key cell types including pancreatic exocrine cells, islet cells, immune cells, and stromal components. We employed this panel to analyze 12 pancreata obtained from donors with clinically diagnosed T1D and 6 pancreata from non-diabetic controls. In the pancreata from donors with T1D, we simultaneously visualized significant alterations in islet architecture, endocrine cell composition, and immune cell presentation. Indeed, we demonstrate the utility of IMC to investigate complex events on the cellular level that will provide new insights on the pathophysiology of T1D.
Keywords: T1D; histopathology; human pancreas; imaging; imaging mass cytometry; immune cell composition; immunolabeling; islet structure; multiplexed imaging; spatial information; type 1 diabetes.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare that no conflicts of interest exist pertaining to the contents of this manuscript.
Figures




Comment in
-
The reconstructed natural history of type 1 diabetes mellitus.Nat Rev Endocrinol. 2019 May;15(5):256-257. doi: 10.1038/s41574-019-0192-8. Nat Rev Endocrinol. 2019. PMID: 30858529 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

