Detection and identification of solids, surfaces, and solutions of uranium using vibrational spectroscopy
- PMID: 30713345
- PMCID: PMC6358285
- DOI: 10.1016/j.ccr.2018.07.010
Detection and identification of solids, surfaces, and solutions of uranium using vibrational spectroscopy
Abstract
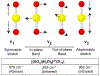
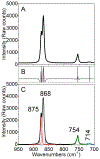
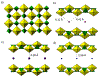
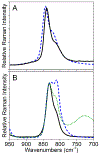
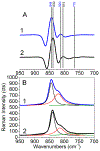
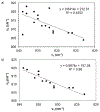
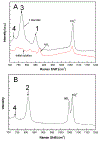
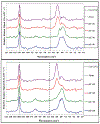
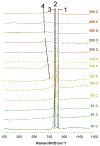
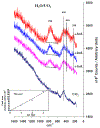
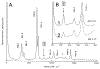
The purpose of this review is to provide an overview of uranium speciation using vibrational spectroscopy methods including Raman and IR. Uranium is a naturally occurring, radioactive element that is utilized in the nuclear energy and national security sectors. Fundamental uranium chemistry is also an active area of investigation due to ongoing questions regarding the participation of 5f orbitals in bonding, variation in oxidation states and coordination environments, and unique chemical and physical properties. Importantly, uranium speciation affects fate and transportation in the environment, influences bioavailability and toxicity to human health, controls separation processes for nuclear waste, and impacts isotopic partitioning and geochronological dating. This review article provides a thorough discussion of the vibrational modes for U(IV), U(V), and U(VI) and applications of infrared absorption and Raman scattering spectroscopies in the identification and detection of both naturally occurring and synthetic uranium species in solid and solution states. The vibrational frequencies of the uranyl moiety, including both symmetric and asymmetric stretches are sensitive to the coordinating ligands and used to identify individual species in water, organic solvents, and ionic liquids or on the surface of materials. Additionally, vibrational spectroscopy allows for the in situ detection and real-time monitoring of chemical reactions involving uranium. Finally, techniques to enhance uranium species signals with vibrational modes are discussed to expand the application of vibrational spectroscopy to biological, environmental, inorganic, and materials scientists and engineers.
Keywords: Infrared; Raman; Uranium; Vibrational spectroscopy.
Figures




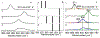
References
-
- Lide DR, in: CRC Handbook of Chemistry and Physics, Taylor & Francis Group, Boca Raton, FL, 2008.
-
- Choppin G, Liljenzin J-O, Rydberg J, Ekberg C, Radiochemistry and Nuclear Chemistry, fourth ed., Academic Press, Oxford, 2013.
-
- Bruno J, Ewing RC, Elements 2 (2006) 343–349.
-
- Macfarlane AM, Ewing RC, in: Uncertainty Underground, MIT Press, Cambridge, MA, 2006.
-
- Abdelouas A, Elements 2 (2006) 335–341.
Grants and funding
LinkOut - more resources
Full Text Sources
