A Novel T-Cell Engaging Bi-specific Antibody Targeting the Leukemia Antigen PR1/HLA-A2
- PMID: 30713535
- PMCID: PMC6345694
- DOI: 10.3389/fimmu.2018.03153
A Novel T-Cell Engaging Bi-specific Antibody Targeting the Leukemia Antigen PR1/HLA-A2
Abstract
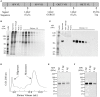
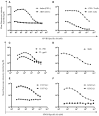
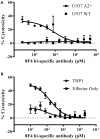
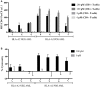
Despite substantial advances in the treatment of acute myeloid leukemia (AML), only 30% of patients survive more than 5 years. Therefore, new therapeutics are much needed. Here, we present a novel therapeutic strategy targeting PR1, an HLA-A2 restricted myeloid leukemia antigen. Previously, we have developed and characterized a novel T-cell receptor-like monoclonal antibody (8F4) that targets PR1/HLA-A2 and eliminates AML xenografts by antibody-dependent cellular cytotoxicity (ADCC). To improve the potency of 8F4, we adopted a strategy to link T-cell cytotoxicity with a bi-specific T-cell-engaging antibody that binds PR1/HLA-A2 on leukemia and CD3 on neighboring T-cells. The 8F4 bi-specific antibody maintained high affinity and specific binding to PR1/HLA-A2 comparable to parent 8F4 antibody, shown by flow cytometry and Bio-Layer Interferometry. In addition, 8F4 bi-specific antibody activated donor T-cells in the presence of HLA-A2+ primary AML blasts and cell lines in a dose dependent manner. Importantly, activated T-cells lysed HLA-A2+ primary AML blasts and cell lines after addition of 8F4 bi-specific antibody. In conclusion, our studies demonstrate the therapeutic potential of a novel bi-specific antibody targeting the PR1/HLA-A2 leukemia-associated antigen, justifying further clinical development of this strategy.
Keywords: PR1; acute myeloid leukemia; bi-specific antibody; cancer immunotherapy; re-directed cytotoxicity.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

