IgG Fc Glycosylation Patterns of Preterm Infants Differ With Gestational Age
- PMID: 30713537
- PMCID: PMC6346593
- DOI: 10.3389/fimmu.2018.03166
IgG Fc Glycosylation Patterns of Preterm Infants Differ With Gestational Age
Abstract
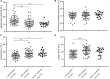
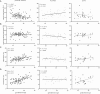
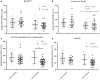
Preterm infants acquire reduced amounts of Immunoglobulin G (IgG) via trans-placental transfer as compared to term infants which might explain their high susceptibility for infections. The reduced amount of IgG antibodies also results in a lower amount of anti-inflammatory Fc N-galactosylated and -sialylated IgG antibodies. This reduction or, even more, a qualitative shift in the type of IgG Fc glycosylation might contribute to the increased risk for sustained inflammatory diseases in preterm infants. It was the aim of our explorative study to investigate the IgG Fc glycosylation patterns in preterm infants of different gestational ages compared to term infants and mothers of preterm infants. In plasma samples of preterm infants (n = 38), we investigated IgG concentrations by use of ELISA. Furthermore, we analyzed IgG Fc glycosylation patterns in plasma of preterm infants (n = 86, 23-34 weeks of gestation), term infants (n = 15) and mothers from preterm infants (n = 41) using high performance liquid chromatography. Extremely low gestational age infants (born < 28 weeks of gestation during second trimester) had reduced IgG concentrations and decreased proportions of galactosylated (84.5 vs. 88.4%), sialylated (14.5 vs. 17.9%) and bisecting N-acetylglucosamine-containing (8.4 vs. 10.8%) IgG Fc N-linked glycans as compared to preterm infants born ≥28 weeks of gestation (during third trimester) and term infants. Increased non-galactosylated (agalactosylated, 16.9 vs. 10.6%) IgG Fc N-linked glycans were associated with the development of chronic inflammatory bronchopulmonary dysplasia (BPD). However, mothers of preterm infants born during second or third trimester of pregnancy did not show significant differences in IgG Fc glycosylation patterns. Thus, the IgG Fc glycosylation patterns of preterm infants depend on their gestational age. Although lack of bisecting N-acetylglucosamine has been associated with less inflammatory effector functions, the decreased IgG Fc galactosylation and sialylation with lower gestational age suggest a rather pro-inflammatory pattern. The difference in IgG Fc glycosylation patterns between preterm infants and mothers of preterm infants suggests a selective enrichment of IgG glyco forms in preterm infants, which might contribute to or result of the development of sustained inflammatory diseases like BPD.
Keywords: IgG Fc glycosylation; IgG antibodies; galactosylation; mothers; newborn; preterm infants; sialylation; trans-placental transfer.
Figures


References
-
- Morell A, Skvaril F, Hitzig WH, Barandun S. IgG subclasses: development of the serum concentrations in ‘normal' infants and children. J Pediatr. (1972) 80:960–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

