A functional interplay between intein and extein sequences in protein splicing compensates for the essential block B histidine
- PMID: 30713635
- PMCID: PMC6333167
- DOI: 10.1039/c8sc01074a
A functional interplay between intein and extein sequences in protein splicing compensates for the essential block B histidine
Abstract
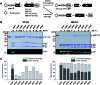
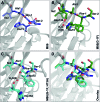
Inteins remove themselves from a precursor protein by protein splicing. Due to the concomitant structural changes of the host protein, this self-processing reaction has enabled many applications in protein biotechnology and chemical biology. We show that the evolved M86 mutant of the Ssp DnaB intein displays a significantly improved tolerance towards non-native amino acids at the N-terminally flanking (-1) extein position compared to the parent intein, in the form of both an artificially trans-splicing split intein and the cis-splicing mini-intein. Surprisingly, side chains with increased steric bulk compared to the native Gly(-1) residue, including d-amino acids, were found to compensate for the essential block B histidine in His73Ala mutants in the initial N-S acyl shift of the protein splicing pathway. In the case of the M86 intein, large (-1) side chains can even rescue protein splicing activity as a whole. With the comparison of three crystal structures, namely of the M86 intein as well as of its Gly(-1)Phe and Gly(-1)Phe/His73Ala mutants, our data supports a model in which the intein's active site can exert a strain by varying mechanisms on the different angles of the scissile bond at the extein-intein junction to effect a ground-state destabilization. The compensatory mechanism of the block B histidine is the first example for the direct functional role of an extein residue in protein splicing. It sheds new light on the extein-intein interplay and on possible consequences of their co-evolution as well as on the laboratory engineering of improved inteins.
Figures







References
-
- Hirata R., Ohsumk Y., Nakano A., Kawasaki H., Suzuki K., Anraku Y. J. Biol. Chem. 1990;265:6726–6733. - PubMed
-
- Kane P. M., Yamashiro C. T., Wolczyk D. F., Neff N., Goebl M., Stevens T. H. Science. 1990;250:651–657. - PubMed
-
- Noren C. J., Wang J., Perler F. B. Angew. Chem., Int. Ed. Engl. 2000;39:450–466. - PubMed
-
- Volkmann G., Iwai H. Mol. BioSyst. 2010;6:2110–2121. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

