Stereoselective organocatalyzed glycosylations - thiouracil, thioureas and monothiophthalimide act as Brønsted acid catalysts at low loadings
- PMID: 30713648
- PMCID: PMC6334493
- DOI: 10.1039/c8sc02788a
Stereoselective organocatalyzed glycosylations - thiouracil, thioureas and monothiophthalimide act as Brønsted acid catalysts at low loadings
Abstract
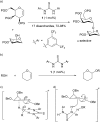
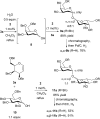
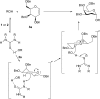
Thiouracil catalyzes stereoselective glycosylations with galactals in loadings as low as 0.1 mol%. It is proposed that in these glycosylations thiouracil, monothiophthalimide, and the previously reported catalyst, Schreiner's thiourea, do not operate via a double H-bonding mechanism but rather by Brønsted acid/base catalysis. In addition to the synthesis of 2-deoxyglycosides and glycoconjugates, we report the first organocatalytic synthesis of 1,1'-linked trehalose-type sugars.
Figures




References
-
- Jakab G. and Schreiner P. R. in Comprehensive Enantioselective Organocatalysis, ed. P. Dalko, Wiley-VCH, Weinheim, 2013, ch. 12, vol. 2.
- Hof K., Lippert K. M. and Schreiner P. R. in Asymmetric Organocatalysis, ed. K. Maruoka), Thieme, Stuttgart, 2012, ch. 2.2.4, vol. 2.
- Doyle A. G., Jacobsen E. N. Chem. Rev. 2007;107:5713. - PubMed
- Hamza A., Schubert G., Soós T., Pápai I. J. Am. Chem. Soc. 2006;128:13151. - PubMed
- Okino T., Hoashi Y., Takemoto Y. J. Am. Chem. Soc. 2003;125:12672. - PubMed
- Schreiner P. R., Wittkopp A. Org. Lett. 2002;4:217. - PubMed
- Zuend S. J., Jacobsen E. N. J. Am. Chem. Soc. 2007;129:15872. - PubMed
- Zuend S. J., Jacobsen E. N. J. Am. Chem. Soc. 2009;131:15358. - PMC - PubMed
-
-
. A thiourea catalyst with one NH was recently described: . See also . For proposed action of thioureas as Lewis bases see:
- Madarász Á., Dósa Z., Varga S., Soós T., Csámpai A., Pápai I. ACS Catal. 2016;6:4379.
- Madarász Á., Dósa Z., Varga S., Soós T., Csámpai A., and Pápai I., oral presentation, EU Cost Action CM0905 Meeting, Palermo, May 2014.
- Jovanovic P., Petkovic M., Simic M., Ivkovic B., Savic V. Org. Biomol. Chem. 2016;14:6712. - PubMed
- Supady A., Hecht S., Baldau C. Org. Lett. 2017;19:4199. - PubMed
- Tan C. K., Chen F., Yeung Y.-Y. Tetrahedron Lett. 2011;52:4892.
- Tripathi C. B., Mukherjee S. J. Org. Chem. 2012;77:1592. - PubMed
- Denmark S. E., Burk M. T. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20655. - PMC - PubMed
- Kobayashi Y., Nakatsuji Y., Li S., Tsukuki S., Takemoto Y. Angew. Chem., Int. Ed. 2018;57:3646. - PubMed
-
-
- Balmond E. I., Coe D. M., Galan M. C., McGarrigle E. M. Angew. Chem., Int. Ed. 2012;51:9152. - PubMed
-
- Kuehle E., Eue L. and Bayer O., BE 613427, 1962, CA59:8698.
- Balmond E. I., Galan M. C., and McGarrigle E. M., N,N′-Bis[3,5-bis(trifluoromethyl)phenyl]-thiourea, in Encyclopedia of Reagents for Organic Synthesis, online, ed. L. A. Paquette, D. Crich, P. L. Fuchs, and G. A. Molander, John Wiley & Sons Ltd., 2014, 10.1002/047084289X.rn01642. - DOI
- Zhang Z., Bao Z., Xing H. Org. Biomol. Chem. 2014;12:3151. - PubMed
-
-
For leading references on organocatalytic glycosylations see:
- Cox D. J., Smith M. D., Fairbanks A. J. Org. Lett. 2010;12:1452. - PubMed
- Balmond E. I., Galan M. C., McGarrigle E. M. Synlett. 2013;24:2335.
- Geng Y. Q., Kumar A., Faidallah H. M., Albar H. A., Mhkalid I. A., Schmidt R. R. Angew. Chem., Int. Ed. 2013;52:10089. - PubMed
- Das S., Pekel D., Neudorfl J. M., Berkessel A. Angew. Chem., Int. Ed. 2015;54:12479. - PubMed
- Schmalisch S., Mahrwald R. Org. Lett. 2013;15:5854. - PubMed
- Sun L., Wu X., Xiong D.-C., Ye X.-S. Angew. Chem., Int. Ed. 2016;55:8041. - PubMed
- Tanaka M., Nashida J., Takahashi D., Toshima K. Org. Lett. 2016;18:2288. - PubMed
- Gouliaras C., Lee D., Chan L., Taylor M. S. J. Am. Chem. Soc. 2011;133:13926. - PubMed
- Medina S., Harper M., Balmond E. I., Miranda S., Crisenza G., Coe D. M., McGarrigle E. M., Galan M. C. Org. Lett. 2016;18:4222. - PMC - PubMed
- Park Y., Harper K. C., Kuhl N., Kwan E. E., Liu R. Y., Jacobsen E. N. Science. 2017;355:162. - PMC - PubMed
- Williams R., Galan M. C. Eur. J. Org. Chem. 2017:6247. - PMC - PubMed
- Liu J.-L., Zhang Y.-T., Liu H.-F., Zhou L., Chen J. Org. Lett. 2017;19:5272. - PubMed
- Lee J., Borovika A., Khomutnyk Y., Nagorny P. Chem. Commun. 2017;53:8976. - PubMed
- Palo-Nieto C., Sau A., Williams R., Galan M. C. J. Org. Chem. 2017;82:407. - PMC - PubMed
- Kimura T., Eto T., Takahashi D., Toshima K. Org. Lett. 2016;18:3190. - PubMed
- Shaw M., Kumar Y., Thakur R., Kumar A. Beilstein J. Org. Chem. 2017;13:2385. - PMC - PubMed
- Singh Y., Wang T., Geringer S. A., Stine K. J., Demchenko A. V. J. Org. Chem. 2018;83:374. - PMC - PubMed
- Tay J.-H., Argüelles A. J., DeMars M. D., Zimmerman P. M., Sherman D. H., Nagorny P. J. Am. Chem. Soc. 2017;139:8570. - PMC - PubMed
-
LinkOut - more resources
Full Text Sources

