Tofacitinib or adalimumab versus placebo: patient-reported outcomes from OPAL Broaden-a phase III study of active psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs
- PMID: 30713721
- PMCID: PMC6340575
- DOI: 10.1136/rmdopen-2018-000806
Tofacitinib or adalimumab versus placebo: patient-reported outcomes from OPAL Broaden-a phase III study of active psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs
Abstract
Objectives: Tofacitinib is an oral Janus kinase inhibitor for treatment of psoriatic arthritis (PsA). We evaluated patient-reported outcomes (PROs) in patients with PsA refractory to ≥1 conventional synthetic disease-modifying antirheumatic drug (csDMARD-IR) and tumour necrosis factor inhibitor-naïve in a 12-month, phase III randomised controlled trial (OPAL Broaden [NCT01877668]).
Methods: Patients (N=422) received tofacitinib 5 mg or 10 mg twice daily, adalimumab 40 mg subcutaneously every 2 weeks or placebo advancing to tofacitinib 5 mg or 10 mg twice daily at month 3. Least squares mean changes from baseline and percentages of patients reporting improvements ≥minimum clinically important differences (MCID); and scores ≥normative values in: Patient Global Assessment of disease activity (PtGA), Pain, Patient Global Joint and Skin Assessment (PGJS), Short Form-36 Health Survey version 2 (SF-36v2), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue), EuroQol 5-Dimensions-3-level questionnaire (EQ-5D-3L) and Ankylosing Spondylitis Quality of Life (ASQoL) were determined. Nominal p values were cited without multiple comparison adjustments.
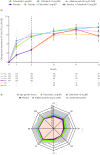
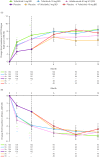
Results: At month 3, PtGA, Pain, PGJS, FACIT-Fatigue, EQ-5D-3L, ASQoL and SF-36v2 Physical Component Summary (PCS), physical functioning (PF), bodily pain (BP) and vitality domain scores exceeded placebo with both tofacitinib doses (p≤0.05); SF-36v2 social functioning with 5 mg twice daily (p≤0.05). Percentages reporting improvements ≥MCID in PtGA, Pain, PGJS, FACIT-Fatigue, ASQoL and SF-36v2 PCS, PF, BP and general health scores exceeded placebo with both tofacitinib doses (p≤0.05) and were similar with adalimumab.
Conclusion: csDMARD-IR patients with active PsA reported statistically and clinically meaningful improvements in PROs with tofacitinib compared with placebo at Month 3.
Keywords: DMARDs (synthetic); outcomes research; patient perspective; psoriatic arthritis; treatment.
Conflict of interest statement
Competing interests: VS has received consulting fees from AbbVie, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Genentech/Roche, GSK, Janssen, Lilly, Merck, Novartis, Pfizer Inc, Regeneron, Samsung, Sandoz, Sanofi and UCB. KdV is a consultant and advisory board member for Pfizer Inc. JAC-C is an investigator for Pfizer Inc. PJM has received research grants from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, Pfizer Inc, Sun and UCB; has acted as a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Janssen, Lilly, Merck, Novartis, Pfizer Inc, Sun, UCB and Zynerba; and has participated in speakers’ bureaus for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Novartis, Pfizer Inc and UCB. DDG has received research grants from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, Pfizer Inc and UCB and has acted as a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, Pfizer Inc and UCB. M-AH, DG, CW and JCC are shareholders and employees of Pfizer Inc. TH is a shareholder, and was an employee, of Pfizer Inc during the time of this analysis.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
