β-adrenergic-mediated dynamic augmentation of sarcolemmal CaV 1.2 clustering and co-operativity in ventricular myocytes
- PMID: 30714156
- PMCID: PMC6462464
- DOI: 10.1113/JP277283
β-adrenergic-mediated dynamic augmentation of sarcolemmal CaV 1.2 clustering and co-operativity in ventricular myocytes
Abstract
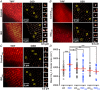
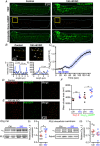
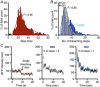
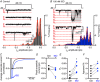
Key points: Prevailing dogma holds that activation of the β-adrenergic receptor/cAMP/protein kinase A signalling pathway leads to enhanced L-type CaV 1.2 channel activity, resulting in increased Ca2+ influx into ventricular myocytes and a positive inotropic response. However, the full mechanistic and molecular details underlying this phenomenon are incompletely understood. CaV 1.2 channel clusters decorate T-tubule sarcolemmas of ventricular myocytes. Within clusters, nanometer proximity between channels permits Ca2+ -dependent co-operative gating behaviour mediated by physical interactions between adjacent channel C-terminal tails. We report that stimulation of cardiomyocytes with isoproterenol, evokes dynamic, protein kinase A-dependent augmentation of CaV 1.2 channel abundance along cardiomyocyte T-tubules, resulting in the appearance of channel 'super-clusters', and enhanced channel co-operativity that amplifies Ca2+ influx. On the basis of these data, we suggest a new model in which a sub-sarcolemmal pool of pre-synthesized CaV 1.2 channels resides in cardiomyocytes and can be mobilized to the membrane in times of high haemodynamic or metabolic demand, to tune excitation-contraction coupling.
Abstract: Voltage-dependent L-type CaV 1.2 channels play an indispensable role in cardiac excitation-contraction coupling. Activation of the β-adrenergic receptor (βAR)/cAMP/protein kinase A (PKA) signalling pathway leads to enhanced CaV 1.2 activity, resulting in increased Ca2+ influx into ventricular myocytes and a positive inotropic response. CaV 1.2 channels exhibit a clustered distribution along the T-tubule sarcolemma of ventricular myocytes where nanometer proximity between channels permits Ca2+ -dependent co-operative gating behaviour mediated by dynamic, physical, allosteric interactions between adjacent channel C-terminal tails. This amplifies Ca2+ influx and augments myocyte Ca2+ transient and contraction amplitudes. We investigated whether βAR signalling could alter CaV 1.2 channel clustering to facilitate co-operative channel interactions and elevate Ca2+ influx in ventricular myocytes. Bimolecular fluorescence complementation experiments reveal that the βAR agonist, isoproterenol (ISO), promotes enhanced CaV 1.2-CaV 1.2 physical interactions. Super-resolution nanoscopy and dynamic channel tracking indicate that these interactions are expedited by enhanced spatial proximity between channels, resulting in the appearance of CaV 1.2 'super-clusters' along the z-lines of ISO-stimulated cardiomyocytes. The mechanism that leads to super-cluster formation involves rapid, dynamic augmentation of sarcolemmal CaV 1.2 channel abundance after ISO application. Optical and electrophysiological single channel recordings confirm that these newly inserted channels are functional and contribute to overt co-operative gating behaviour of CaV 1.2 channels in ISO stimulated myocytes. The results of the present study reveal a new facet of βAR-mediated regulation of CaV 1.2 channels in the heart and support the novel concept that a pre-synthesized pool of sub-sarcolemmal CaV 1.2 channel-containing vesicles/endosomes resides in cardiomyocytes and can be mobilized to the sarcolemma to tune excitation-contraction coupling to meet metabolic and/or haemodynamic demands.
Keywords: L-type calcium channels; coupled gating; β-adrenergic receptors.
© 2019 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures



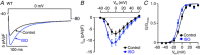

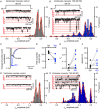
Comment in
-
Channel surfing: new insights into plasticity of excitation-contraction coupling.J Physiol. 2019 Apr;597(8):2119-2120. doi: 10.1113/JP277783. Epub 2019 Mar 20. J Physiol. 2019. PMID: 30854650 Free PMC article. No abstract available.
References
-
- Altier C, Garcia‐Caballero A, Simms B, You H, Chen L, Walcher J, Tedford HW, Hermosilla T & Zamponi GW (2011). The CaVβ subunit prevents RFP2‐mediated ubiquitination and proteasomal degradation of L‐type channels. Nat Neurosci 14, 173–180. - PubMed
-
- Bean BP, Nowycky MC & Tsien RW (1984). Beta‐adrenergic modulation of calcium channels in frog ventricular heart cells. Nature 307, 371–375. - PubMed
-
- Bichet D, Cornet V, Geib S, Carlier E, Volsen S, Hoshi T, Mori Y & De Waard M (2000). The I‐II loop of the Ca2+ channel α1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron 25, 177–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

