Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration
- PMID: 30714354
- PMCID: PMC7654553
- DOI: 10.1002/adhm.201801217
Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration
Abstract
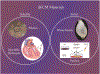
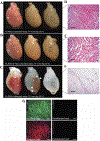
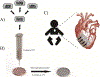
Decellularized extracellular matrix (dECM) is a promising biomaterial for repairing cardiovascular tissue, as dECM most effectively captures the complex array of proteins, glycosaminoglycans, proteoglycans, and many other matrix components that are found in native tissue, providing ideal cues for regeneration and repair of damaged myocardium. dECM can be used in a variety of forms, such as solid scaffolds that maintain native matrix structure, or as soluble materials that can form injectable hydrogels for tissue repair. dECM has found recent success in many regeneration and repair therapies, such as for musculoskeletal, neural, and liver tissues. This review focuses on dECM in the context of cardiovascular applications, with variations in tissue and species sourcing, and specifically discusses advances in solid and soluble dECM development, in vitro studies, in vivo implementation, and clinical translation.
Keywords: cardiac patches; decellularized; extracellular matrices; injectable hydrogels.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures





References
-
- Taylor DA, Sampaio LC, Ferdous Z, Gobin AS, Taite LJ, Acta Biomater. 2018, 74, 74. - PubMed
-
- Garreta E, Oria R, Tarantino C, Pla-Roca M, Prado P, Fernandez-Aviles F, Campistol JM, Samitier J, Montserrat N, Mater. Today 2017, 20, 166.
-
- Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J, Circulation 2008, 117, 1388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

