A Bidirectional Neuromodulation Technology for Nerve Recording and Stimulation
- PMID: 30715037
- PMCID: PMC6267106
- DOI: 10.3390/mi9110538
A Bidirectional Neuromodulation Technology for Nerve Recording and Stimulation
Abstract
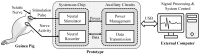
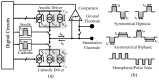
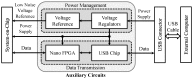
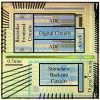
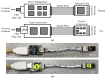
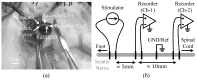
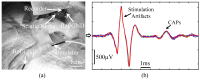
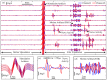
Electrical nerve recording and stimulation technologies are critically needed to monitor and modulate nerve activity to treat a variety of neurological diseases. However, current neuromodulation technologies presented in the literature or commercially available products cannot support simultaneous recording and stimulation on the same nerve. To solve this problem, a new bidirectional neuromodulation system-on-chip (SoC) is proposed in this paper, which includes a frequency-shaping neural recorder and a fully integrated neural stimulator with charge balancing capability. In addition, auxiliary circuits consisting of power management and data transmission circuits are designed to provide the necessary power supply for the SoC and the bidirectional data communication between the SoC and an external computer via a universal serial bus (USB) interface, respectively. To achieve sufficient low input noise for sensing nerve activity at a sub-10 μ V range, several noise reduction techniques are developed in the neural recorder. The designed SoC was fabricated in a 0.18 μ m high-voltage Bipolar CMOS DMOS (BCD) process technology that was described in a previous publication and it has been recently tested in animal experiments that demonstrate the proposed SoC is capable of achieving reliable and simultaneous electrical stimulation and recording on the same nerve.
Keywords: bidirectional; closed-loop; neuromodulation; precision medicine; sciatic nerve; system-on-chip; vagus nerve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

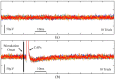
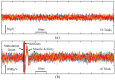
References
-
- Burns J., Hsieh Y.H., Mueller A., Chevallier J., Sriram T.S., Lewis S.J., Chew D., Achyuta A., Fiering J. High density penetrating electrode arrays for autonomic nerves; Proceedings of the 38th Annual International of the IEEE EMBS Conference; Orlando, FL, USA. 17–20 August 2016; pp. 2802–2805. - PubMed
-
- Abdelhalim K., Kokarovtseva L., Velazquez J., Genov R. 915-MHz FSK/OOK Wireless Neural Recording SoC With 64 Mixed-Signal FIR Filters. IEEE J. Solid-State Circuits. 2013;48:2478–2493. doi: 10.1109/JSSC.2013.2272849. - DOI
-
- Biederman W., Yeager D., Narevsky N., Koralek A., Carmena J., Alon E., Rabaey J. A Fully Integrated, Miniaturized (0.125 mm2) 10.5 μW Wireless Neural Sensor. IEEE J. Solid-State Circuits. 2013;48:960–970. doi: 10.1109/JSSC.2013.2238994. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

