Biocatalysis by Transglutaminases: A Review of Biotechnological Applications
- PMID: 30715061
- PMCID: PMC6265872
- DOI: 10.3390/mi9110562
Biocatalysis by Transglutaminases: A Review of Biotechnological Applications
Abstract
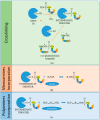
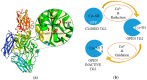
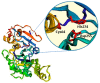
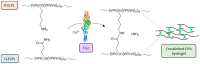
The biocatalytic activity of transglutaminases (TGs) leads to the synthesis of new covalent isopeptide bonds (crosslinks) between peptide-bound glutamine and lysine residues, but also the transamidation of primary amines to glutamine residues, which ultimately can result into protein polymerisation. Operating with a cysteine/histidine/aspartic acid (Cys/His/Asp) catalytic triad, TGs induce the post-translational modification of proteins at both physiological and pathological conditions (e.g., accumulation of matrices in tissue fibrosis). Because of the disparate biotechnological applications, this large family of protein-remodelling enzymes have stimulated an escalation of interest. In the past 50 years, both mammalian and microbial TGs polymerising activity has been exploited in the food industry for the improvement of aliments' quality, texture, and nutritive value, other than to enhance the food appearance and increased marketability. At the same time, the ability of TGs to crosslink extracellular matrix proteins, like collagen, as well as synthetic biopolymers, has led to multiple applications in biomedicine, such as the production of biocompatible scaffolds and hydrogels for tissue engineering and drug delivery, or DNA-protein bio-conjugation and antibody functionalisation. Here, we summarise the most recent advances in the field, focusing on the utilisation of TGs-mediated protein multimerisation in biotechnological and bioengineering applications.
Keywords: biomedicine; crosslinking; food industry; polymerisation; transglutaminases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Demeny M.A., Korponay-Szabo I., Fesus L. Structure of Transglutaminases: Unique Features Serve Diverse Functions. In: Hitomi K., Kojima S., Fesus L., editors. Transglutaminases: Multiple Functional Modifiers and Targets for New Drug Discovery. Springer; Tokyo, Japan: 2015. pp. 1–2.
-
- Folk J.E. Mechanism of Action of Guinea Pig Liver Transglutaminase. VI. Order of Substrate Addition. J. Biol. Chem. 1969;244:3707–3713. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

