Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001
- PMID: 30715153
- PMCID: PMC6503622
- DOI: 10.1093/annonc/mdz011
Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001
Abstract
Background: Pembrolizumab demonstrated robust antitumor activity and safety in the phase Ib KEYNOTE-001 study (NCT01295827) of advanced melanoma. Five-year outcomes in all patients and treatment-naive patients are reported herein. Patients whose disease progressed following initial response and who received a second course of pembrolizumab were also analyzed.
Patients and methods: Patients aged ≥18 years with previously treated or treatment-naive advanced/metastatic melanoma received pembrolizumab 2 mg/kg every 3 weeks, 10 mg/kg every 3 weeks, or 10 mg/kg every 2 weeks until disease progression, intolerable toxicity, or patient/investigator decision to withdraw. Kaplan-Meier estimates of overall survival (OS) and progression-free survival (PFS) were calculated. Objective response rate and PFS were based on immune-related response criteria by investigator assessment (data cut-off, September 1, 2017).
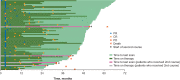
Results: KEYNOTE-001 enrolled 655 patients with melanoma; median follow-up was 55 months. Estimated 5-year OS was 34% in all patients and 41% in treatment-naive patients; median OS was 23.8 months (95% CI, 20.2-30.4) and 38.6 months (95% CI, 27.2-not reached), respectively. Estimated 5-year PFS rates were 21% in all patients and 29% in treatment-naive patients; median PFS was 8.3 months (95% CI, 5.8-11.1) and 16.9 months (95% CI, 9.3-35.5), respectively. Median response duration was not reached; 73% of all responses and 82% of treatment-naive responses were ongoing at data cut-off; the longest response was ongoing at 66 months. Four patients [all with prior response of complete response (CR)] whose disease progressed during observation subsequently received second-course pembrolizumab. One patient each achieved CR and partial response (after data cut-off). Treatment-related AEs (TRAEs) occurred in 86% of patients and resulted in study discontinuation in 7.8%; 17% experienced grade 3/4 TRAE.
Conclusions: This 5-year analysis of KEYNOTE-001 represents the longest follow-up for pembrolizumab to date and confirms the durable antitumor activity and tolerability of pembrolizumab in advanced melanoma.
Clinical trial registry: ClinicalTrials.gov, NCT01295827.
Keywords: long-term follow-up; melanoma; metastatic; overall survival; pembrolizumab; treatment-naive.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures

Comment in
-
Treatment in metastatic melanoma-time to re-think.Ann Oncol. 2019 Apr 1;30(4):501-503. doi: 10.1093/annonc/mdz050. Ann Oncol. 2019. PMID: 30768148 No abstract available.
References
-
- Corrie P, Hategan M, Fife K, Parkinson C.. Management of melanoma. Br Med Bull 2014; 111(1): 149–162. - PubMed
-
- National Comprehensive Cancer Network I. NCCN Clinical Practice Guidelines in Oncology – Melanoma, v3.2018. In 3.2018 (ed) Version 3.2018, July 12, 2018 Edition; https://www.nccn.org/professionals/physician_gls/default.aspx (30 January 2019, date last accessed).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

