Cardiovascular toxicities associated with immune checkpoint inhibitors
- PMID: 30715219
- PMCID: PMC6452314
- DOI: 10.1093/cvr/cvz026
Cardiovascular toxicities associated with immune checkpoint inhibitors
Erratum in
-
Corrigendum to: Cardiovascular toxicities associated with immune checkpoint inhibitors.Cardiovasc Res. 2019 Apr 15;115(5):868. doi: 10.1093/cvr/cvz082. Cardiovasc Res. 2019. PMID: 30958559 Free PMC article. No abstract available.
Abstract
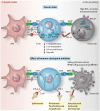
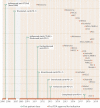
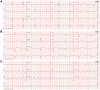
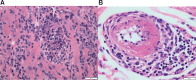
Cardiovascular toxicities associated with immune checkpoint inhibitors (ICIs) have been reported in case series but have been underappreciated due to their recent emergence, difficulties in diagnosis and non-specific clinical manifestations. ICIs are antibodies that block negative regulators of the T cell immune response, including cytotoxic T lymphocyte-associated protein-4 (CTLA-4), programmed cell death protein-1 (PD-1), and PD-1 ligand (PD-L1). While ICIs have introduced a significant mortality benefit in several cancer types, the augmented immune response has led to a range of immune-related toxicities, including cardiovascular toxicity. ICI-associated myocarditis often presents with arrhythmias, may co-exist with myositis and myasthenia gravis, can be severe, and portends a poor prognosis. In addition, pericardial disease, vasculitis, including temporal arteritis, and non-inflammatory heart failure, have been recently described as immune-related toxicities from ICI. This narrative review describes the epidemiology, diagnosis, pathophysiology, and treatment of cardiovascular toxicities of ICI therapy, highlighting recent developments in the field in the past year.
Keywords: Cardio-oncology; Cardiovascular toxicity; Immune checkpoint inhibitors; Myocarditis; Pericarditis; Vasculitis.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2019. For permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L.. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4: 127ra37. - PMC - PubMed
-
- Johnson DB, Chandra S, Sosman JA.. Immune checkpoint inhibitor toxicity in 2018. JAMA 2018;320:1702–1703. - PubMed
-
- Friedman CF, Proverbs-Singh TA, Postow MA.. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016;2:1346–1353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

