Cardiomyopathy with lethal arrhythmias associated with inactivation of KLHL24
- PMID: 30715372
- PMCID: PMC6812045
- DOI: 10.1093/hmg/ddz032
Cardiomyopathy with lethal arrhythmias associated with inactivation of KLHL24
Abstract
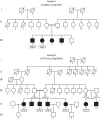
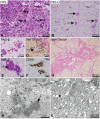
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiovascular disorder, yet the genetic cause of up to 50% of cases remains unknown. Here, we show that mutations in KLHL24 cause HCM in humans. Using genome-wide linkage analysis and exome sequencing, we identified homozygous mutations in KLHL24 in two consanguineous families with HCM. Of the 11 young affected adults identified, 3 died suddenly and 1 had a cardiac transplant due to heart failure. KLHL24 is a member of the Kelch-like protein family, which acts as substrate-specific adaptors to Cullin E3 ubiquitin ligases. Endomyocardial and skeletal muscle biopsies from affected individuals of both families demonstrated characteristic alterations, including accumulation of desmin intermediate filaments. Knock-down of the zebrafish homologue klhl24a results in heart defects similar to that described for other HCM-linked genes providing additional support for KLHL24 as a HCM-associated gene. Our findings reveal a crucial role for KLHL24 in cardiac development and function.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





References
-
- Sen-Chowdhry S., Jacoby D., Moon J.C. and McKenna W.J. (2016) Update on hypertrophic cardiomyopathy and a guide to the guidelines. Nat. Rev. Cardiol., 13, 651–675. - PubMed
-
- Alfares A.A., Kelly M.A., McDermott G., Funke B.H., Lebo M.S., Baxter S.B., Shen J., McLaughlin H.M., Clark E.H., Babb L.J. et al. (2015) Results of clinical genetic testing of 2912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genet. Med., 17, 880–888. - PubMed
-
- Lin Z., Li S., Feng C., Yang S., Wang H., Ma D., Zhang J., Gou M., Bu D., Zhang T. et al. (2016) Stabilizing mutations of KLHL24 ubiquitin ligase cause loss of keratin 14 and human skin fragility. Nat. Genet., 48, 1508–1516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

