Long-term Cross-reactivity Against Nonvaccine Human Papillomavirus Types 31 and 45 After 2- or 3-Dose Schedules of the AS04-Adjuvanted Human HPV-16/18 Vaccine
- PMID: 30715452
- PMCID: PMC6500548
- DOI: 10.1093/infdis/jiy743
Long-term Cross-reactivity Against Nonvaccine Human Papillomavirus Types 31 and 45 After 2- or 3-Dose Schedules of the AS04-Adjuvanted Human HPV-16/18 Vaccine
Abstract
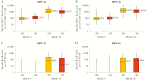
This analysis focused on long-term cross-reactive immunogenicity against nonvaccine human papillomavirus (HPV) types 31 and 45 following 2 doses of AS04-adjuvanted HPV-16/18 vaccine in girls aged 9-14 years or following 3 doses in women aged 15-25 years, for up to 3 years (HPV-070 study) and up to 5 years (HPV-048 study) after the first vaccination. Both schedules elicited antibodies against HPV-31 and HPV-45 up to 5 years after first dose. The antibody concentration was similar in young girls as compared to women. Specific CD4+ T-cell and B-cell responses to HPV-31 and HPV-45 at month 36 were similar across groups. Clinical trials registration: NCT01381575 and NCT00541970.
Keywords: AS04-HPV-16/18 vaccine; HPV-31; HPV-45; cross-reactivity; immunogenicity; nonvaccine types.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- Lehtinen M, Paavonen J, Wheeler CM, et al. ; HPV PATRICIA Study Group Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89–99. - PubMed
-
- de Sanjose S, Quint WG, Alemany L, et al. ; Retrospective International Survey and HPV Time Trends Study Group Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–56. - PubMed
-
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017. Wkly Epidemiol Rec 2017; 19: 241–68. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

