PrimPol is required for the maintenance of efficient nuclear and mitochondrial DNA replication in human cells
- PMID: 30715459
- PMCID: PMC6486543
- DOI: 10.1093/nar/gkz056
PrimPol is required for the maintenance of efficient nuclear and mitochondrial DNA replication in human cells
Abstract
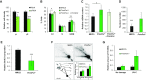
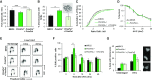
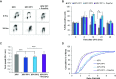
Eukaryotic Primase-Polymerase (PrimPol) is an enzyme that maintains efficient DNA duplication by repriming replication restart downstream of replicase stalling lesions and structures. To elucidate the cellular requirements for PrimPol in human cells, we generated PrimPol-deleted cell lines and show that it plays key roles in maintaining active replication in both the nucleus and mitochondrion, even in the absence of exogenous damage. Human cells lacking PrimPol exhibit delayed recovery after UV-C damage and increased mutation frequency, micronuclei and sister chromatin exchanges but are not sensitive to genotoxins. PrimPol is also required during mitochondrial replication, with PrimPol-deficient cells having increased mtDNA copy number but displaying a significant decrease in replication. Deletion of PrimPol in XPV cells, lacking functional polymerase Eta, causes an increase in DNA damage sensitivity and pronounced fork stalling after UV-C treatment. We show that, unlike canonical TLS polymerases, PrimPol is important for allowing active replication to proceed, even in the absence of exogenous damage, thus preventing the accumulation of excessive fork stalling and genetic mutations. Together, these findings highlight the importance of PrimPol for maintaining efficient DNA replication in unperturbed cells and its complementary roles, with Pol Eta, in damage tolerance in human cells.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Trakselis M.A., Cranford M.T., Chu A.M.. Coordination and substitution of DNA polymerases in response to genomic obstacles. Chem. Res. Toxicol. 2017; 30:1956–1971. - PubMed
-
- Livneh Z., Ziv O., Shachar S.. Multiple two-polymerase mechanisms in mammalian translesion DNA synthesis. Cell Cycle. 2010; 9:729–735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

