Buparlisib in Patients With Recurrent Glioblastoma Harboring Phosphatidylinositol 3-Kinase Pathway Activation: An Open-Label, Multicenter, Multi-Arm, Phase II Trial
- PMID: 30715997
- PMCID: PMC6553812
- DOI: 10.1200/JCO.18.01207
Buparlisib in Patients With Recurrent Glioblastoma Harboring Phosphatidylinositol 3-Kinase Pathway Activation: An Open-Label, Multicenter, Multi-Arm, Phase II Trial
Abstract
Purpose: Phosphatidylinositol 3-kinase (PI3K) signaling is highly active in glioblastomas. We assessed pharmacokinetics, pharmacodynamics, and efficacy of the pan-PI3K inhibitor buparlisib in patients with recurrent glioblastoma with PI3K pathway activation.
Methods: This study was a multicenter, open-label, multi-arm, phase II trial in patients with PI3K pathway-activated glioblastoma at first or second recurrence. In cohort 1, patients scheduled for re-operation after progression received buparlisib for 7 to 13 days before surgery to evaluate brain penetration and modulation of the PI3K pathway in resected tumor tissue. In cohort 2, patients not eligible for re-operation received buparlisib until progression or unacceptable toxicity. Once daily oral buparlisib 100 mg was administered on a continuous 28-day schedule. Primary end points were PI3K pathway inhibition in tumor tissue and buparlisib pharmacokinetics in cohort 1 and 6-month progression-free survival (PFS6) in cohort 2.
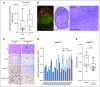
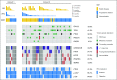
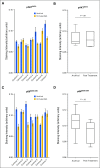
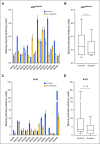
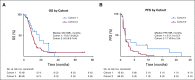
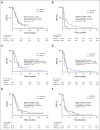
Results: Sixty-five patients were treated (cohort 1, n = 15; cohort 2, n = 50). In cohort 1, reduction of phosphorylated AKTS473 immunohistochemistry score was achieved in six (42.8%) of 14 patients, but effects on phosphoribosomal protein S6S235/236 and proliferation were not significant. Tumor-to-plasma drug level was 1.0. In cohort 2, four (8%) of 50 patients reached 6-month PFS6, and the median PFS was 1.7 months (95% CI, 1.4 to 1.8 months). The most common grade 3 or greater adverse events related to treatment were lipase elevation (n = 7 [10.8%]), fatigue (n = 4 [6.2%]), hyperglycemia (n = 3 [4.6%]), and elevated ALT (n = 3 [4.6%]).
Conclusion: Buparlisib had minimal single-agent efficacy in patients with PI3K-activated recurrent glioblastoma. Although buparlisib achieved significant brain penetration, the lack of clinical efficacy was explained by incomplete blockade of the PI3K pathway in tumor tissue. Integrative results suggest that additional study of PI3K inhibitors that achieve more-complete pathway inhibition may still be warranted.
Trial registration: ClinicalTrials.gov NCT01339052.
Figures


References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA. 2015;314:2535–2543. - PubMed

