CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway
- PMID: 30717785
- PMCID: PMC6360779
- DOI: 10.1186/s13046-019-1049-7
CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway
Abstract
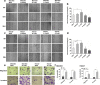
Background: Gastric cancer (GC) has a clear predilection for metastasis toward the omentum which is primarily composed of adipose tissue, indicating that fatty acids may contribute to this phenomenon. However their function remains poorly understood in GC. In this study, we investigated the role of palmitate acid (PA) and its cellular receptor CD36 in the progression of GC.
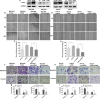
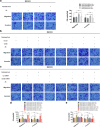
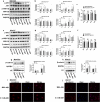
Methods: Immunohistochemical (IHC) staining was performed to detect CD36 expression in GC tissues and its clinical significance was determined statistically. CD36 over-expression and knock-down expression cell models were developed and tested in vitro. Wound-healing assays, migration assays, and invasion assays were performed and peritoneal implants into nude mice were done to assess the biological effects of PA and CD36. The underlying mechanisms were investigated using western blot, immunofluorescence (IF), quantitative real-time PCR (qRT-PCR) and antibody blocking assays.
Results: PA promoted the metastasis of GC by phosphorylation of AKT, which facilitated the nuclear localization of β-catenin through inactivation of GSK-3β via phosphorylation. This tumor-promoting effect of PA was mediated by CD36, a cell surface receptor of fatty acids (FAs). The higher the CD36 expression levels in GC tissues correlated with the poorer the prognosis of patients according to the TCGA database, the GEO database and our own clinical data.
Conclusions: Our experiments established CD36 as a key mediator of FA-induced metastasis of GC via the AKT/GSK-3β/β-catenin signaling pathway. CD36 might, therefore, constitute a potential therapeutic target for clinical intervention in GC.
Keywords: AKT; CD36; GSK-3β; Gastric cancer; Metastasis; Palmitate acid; β-catenin.
Conflict of interest statement
Ethics approval
An institutional review board from the local Human Research Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, China approved the study. All human participants provided informed consent. All animal experiments were also approved by the local Laboratory Animal Ethics Committee of Ruijin Hospital and conducted based on the Guide for the Care and Use Laboratory Animals of Ruijin Hospital. All samples tested for GC were anonymous in accordance with legal and ethical standards.
Consent for publication
All contributing authors agree to the publication of this article.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

