Engineered microbial biofuel production and recovery under supercritical carbon dioxide
- PMID: 30718495
- PMCID: PMC6361901
- DOI: 10.1038/s41467-019-08486-6
Engineered microbial biofuel production and recovery under supercritical carbon dioxide
Abstract
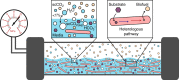
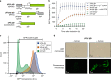
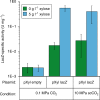
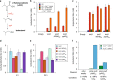
Culture contamination, end-product toxicity, and energy efficient product recovery are long-standing bioprocess challenges. To solve these problems, we propose a high-pressure fermentation strategy, coupled with in situ extraction using the abundant and renewable solvent supercritical carbon dioxide (scCO2), which is also known for its broad microbial lethality. Towards this goal, we report the domestication and engineering of a scCO2-tolerant strain of Bacillus megaterium, previously isolated from formation waters from the McElmo Dome CO2 field, to produce branched alcohols that have potential use as biofuels. After establishing induced-expression under scCO2, isobutanol production from 2-ketoisovalerate is observed with greater than 40% yield with co-produced isopentanol. Finally, we present a process model to compare the energy required for our process to other in situ extraction methods, such as gas stripping, finding scCO2 extraction to be potentially competitive, if not superior.
Conflict of interest statement
J.T.B, A.J.E.F., M.T.T., J.R.T. and K.L.J.P declare a pending patent application for an invention related to this work. The remaining authors declare no competing interests.
Figures

References
-
- Knez Ž, et al. Industrial applications of supercritical fluids: A review. Energy. 2014;77:235–243. doi: 10.1016/j.energy.2014.07.044. - DOI
-
- Darani KK, Mozafari MR. Supercritical fluids technology in bioprocess industries: A review. J. Biochem Tech. 2009;2:144–152.
-
- Salgın U, Salgın S, Takaç S. The enantioselective hydrolysis of racemic naproxen methyl ester in supercritical CO2 using Candida rugosa lipase. J. Supercrit. Fluid. 2007;43:310–316. doi: 10.1016/j.supflu.2007.06.006. - DOI
-
- Matsuda, T., Harada, T. & Nakamura, K. Alcohol dehydrogenase is active in supercritical carbon dioxide. Chem. Commun. 0, 1367–1368 (2000).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

