Generation and characterization of novel anti-DR4 and anti-DR5 antibodies developed by genetic immunization
- PMID: 30718507
- PMCID: PMC6362131
- DOI: 10.1038/s41419-019-1343-5
Generation and characterization of novel anti-DR4 and anti-DR5 antibodies developed by genetic immunization
Abstract
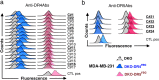
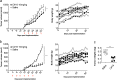
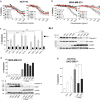
Development of therapeutic antibodies in oncology has attracted much interest in the past decades. More than 30 of them have been approved and are being used to treat patients suffering from cancer. Despite encouraging results, and albeit most clinical trials aiming at evaluating monoclonal antibodies directed against TRAIL agonist receptors have been discontinued, DR4 or DR5 remain interesting targets, since these receptors are overexpressed by tumour cells and are able to trigger their death. In an effort to develop novel and specific anti-DR4 and anti-DR5 antibodies with improved properties, we used genetic immunization to express native proteins in vivo. Injection of DR4 and DR5 cDNA into the tail veins of mice elicited significant humoral anti-DR4 and anti-DR5 responses and fusions of the corresponding spleens resulted in numerous hybridomas secreting antibodies that could specifically recognize DR4 or DR5 in their native forms. All antibodies bound specifically to their targets with a very high affinity, from picomolar to nanomolar range. Among the 21 anti-DR4 and anti-DR5 monoclonal antibodies that we have produced and purified, two displayed proapoptotic properties alone, five induced apoptosis after cross-linking, four were found to potentiate TRAIL-induced apoptosis and three displayed antiapoptotic potential. The most potent anti-DR4 antibody, C#16, was assessed in vivo and was found, alone, to inhibit tumour growth in animal models. This is the first demonstration that DNA-based immunization method can be used to generate novel monoclonal antibodies targeting receptors of the TNF superfamily that may constitute new therapeutic agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

