Endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Pro-inflammatory Signaling in Macrophages and Brain
- PMID: 30718548
- PMCID: PMC6362084
- DOI: 10.1038/s41598-018-37409-6
Endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Pro-inflammatory Signaling in Macrophages and Brain
Abstract
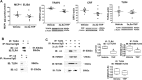
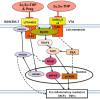
The endogenous neurosteroid (3α,5α)3-hydroxypregnan-20-one (3α,5α-THP, allopregnanolone) has protective activity in animal models of alcoholism, depression, traumatic brain injury, schizophrenia, multiple sclerosis, and Alzheimer's disease that is poorly understood. Because these conditions involve proinflammatory signaling through toll-like receptors (TLRs), we examined the effects of 3α,5α-THP, and pregnenolone on TLR4 activation in both the periphery and the central nervous system (CNS). We used monocytes/macrophages (RAW264.7) as a model of peripheral immune signaling and studied innately activated TLR4 in the ventral tegmental area (VTA) of selectively bred alcohol-preferring (P) rats. LPS activated the TLR4 pathway in RAW264.7 cells as evidenced by increased levels of p-TAK1, TRAF6, NF-κB p50, phospho-NF-κB- p65, pCREB, HMGB1, and inflammatory mediators, including MCP-1 and TNFα. Both 3α,5α-THP and pregnenolone (0.5-1.0μM) substantially (~80%) inhibited these effects, indicating pronounced inhibition of TLR4 signaling. The mechanism of inhibition appears to involve blockade of TLR4/MD-2 protein interactions in RAW246.7 cells. In VTA, 3α,5α-THP (15 mg/kg, IP) administration reduced TRAF6 (~20%), CRF (~30%), and MCP-1 (~20%) levels, as well as TLR4 binding to GABAA receptor α2 subunits (~60%) and MyD88 (~40%). The data suggest that inhibition of proinflammatory neuroimmune signaling underlies protective effects of 3α,5α-THP in immune cells and brain, apparently involving blocking of protein-protein interactions that initiate TLR4-dependent signaling. Inhibition of pro-inflammatory TLR4 activation represents a new mechanism of 3α,5α-THP action in the periphery and the brain.
Conflict of interest statement
The authors have filed for patent protection of the data in this report (Provisional patent #62/674,379). The authors declare no other conflicts of interest.
Figures



References
-
- Selye H. The anesthetic effect of steroid hormones. Proc Soc Exp Biol Med. 1941;46:116–121. doi: 10.3181/00379727-46-11907. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- R01 AA024095/AA/NIAAA NIH HHS/United States
- R01-AA021261/U.S. Department of Health & Human Services | NIH | National Institute on Alcohol Abuse and Alcoholism (NIAAA)/International
- U24 AA015512/AA/NIAAA NIH HHS/United States
- U01-AA020935/U.S. Department of Health & Human Services | NIH | National Institute on Alcohol Abuse and Alcoholism (NIAAA)/International
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

