Three-dimensional organization of transzonal projections and other cytoplasmic extensions in the mouse ovarian follicle
- PMID: 30718581
- PMCID: PMC6362238
- DOI: 10.1038/s41598-018-37766-2
Three-dimensional organization of transzonal projections and other cytoplasmic extensions in the mouse ovarian follicle
Abstract
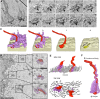
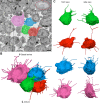
Each mammalian oocyte is nurtured by its own multi-cellular structure, the ovarian follicle. We used new methods for serial section electron microscopy to examine entire cumulus and mural granulosa cells and their projections in mouse antral ovarian follicles. Transzonal projections (TZPs) are thin cytoplasmic projections that connect cumulus cells to the oocyte and are crucial for normal oocyte development. We studied these projections in detail and found that most TZPs do not reach the oocyte, and that they often branch and make gap junctions with each other. Furthermore, the TZPs that connect to the oocyte are usually contacted on their shaft by oocyte microvilli. Mural granulosa cells were found to possess randomly oriented cytoplasmic projections that are strikingly similar to the free-ended TZPs. We propose that granulosa cells use cytoplasmic projections to search for the oocyte, and cumulus cell differentiation results from a contact-mediated paracrine interaction with the oocyte.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

