Phosphoric acid-catalyzed atroposelective construction of axially chiral arylpyrroles
- PMID: 30718716
- PMCID: PMC6361918
- DOI: 10.1038/s41467-019-08447-z
Phosphoric acid-catalyzed atroposelective construction of axially chiral arylpyrroles
Erratum in
-
Publisher Correction: Phosphoric acid-catalyzed atroposelective construction of axially chiral arylpyrroles.Nat Commun. 2019 Apr 26;10(1):2007. doi: 10.1038/s41467-019-10071-w. Nat Commun. 2019. PMID: 31028262 Free PMC article.
Abstract
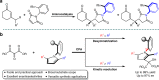
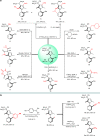
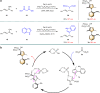
Axially chiral arylpyrroles are key components of pharmaceuticals and natural products as well as chiral catalysts and ligands for asymmetric transformations. However, the catalytic enantioselective construction of optically active arylpyrroles remains a formidable challenge. Here we disclose a highly efficient strategy to access enantioenriched axially chiral arylpyrroles by means of organocatalytic atroposelective desymmetrization and kinetic resolution. Depending on the remote control of chiral catalyst, the arylpyrroles were obtained in high yields and excellent enantioselectivities under mild reaction conditions. This strategy tolerates a wide range of functional groups, providing a facile avenue to approach axially chiral arylpyrroles from simple and readily available starting materials. Selected arylpyrrole products proved to be efficient chiral ligands in asymmetric catalysis and also important precursors for further synthetic transformations into highly functionalized pyrroles with potential bioactivity, especially the axially chiral fully substituted arylpyrroles.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Ito C, Thoyama Y, Omura M, Kajiura I, Furukawa H. Alkaloidal constituents of murraya koenigii. isolation and structural elucidation of novel binary carbazolequinones and carbazole alkaloids. Chem. Pharm. Bull. 1993;41:2096–2100. doi: 10.1248/cpb.41.2096. - DOI
Publication types
LinkOut - more resources
Full Text Sources

