Autoimmune rheumatic disease IgG has differential effects upon neutrophil integrin activation that is modulated by the endothelium
- PMID: 30718722
- PMCID: PMC6361939
- DOI: 10.1038/s41598-018-37852-5
Autoimmune rheumatic disease IgG has differential effects upon neutrophil integrin activation that is modulated by the endothelium
Abstract
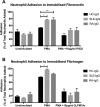
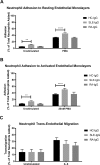
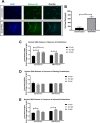
The importance of neutrophils in the pathogenesis of autoimmune rheumatic diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), is increasingly recognised. Generation of reactive oxygen species (ROS) and release of neutrophil extracellular traps (NETs) by activated neutrophils are both thought to contribute to pathology; although the underlying mechanisms, particularly the effects of IgG autoantibodies upon neutrophil function, are not fully understood. Therefore, we determined whether purified IgG from patients with SLE or RA have differential effects upon neutrophil activation and function. We found that SLE- and RA-IgG both bound human neutrophils but differentially regulated neutrophil function. RA- and SLE-IgG both increased PMA-induced β1 integrin-mediated adhesion to fibronectin, whilst only SLE-IgG enhanced αMβ2 integrin-mediated adhesion to fibrinogen. Interestingly, only SLE-IgG modulated neutrophil adhesion to endothelial cells. Both SLE- and RA-IgG increased ROS generation and DNA externalisation by unstimulated neutrophils. Only SLE-IgG however, drove DNA externalisation following neutrophil activation. Co-culture of neutrophils with resting endothelium prevented IgG-mediated increase of extracellular DNA, but this inhibition was overcome for SLE-IgG when the endothelium was stimulated with TNF-α. This differential pattern of neutrophil activation has implications for understanding SLE and RA pathogenesis and may highlight avenues for development of novel therapeutic strategies.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O.Front Immunol. 2021 Mar 4;12:649693. doi: 10.3389/fimmu.2021.649693. eCollection 2021. Front Immunol. 2021. PMID: 33746988 Free PMC article. Review.
-
Caught in a Trap? Proteomic Analysis of Neutrophil Extracellular Traps in Rheumatoid Arthritis and Systemic Lupus Erythematosus.Front Immunol. 2019 Mar 11;10:423. doi: 10.3389/fimmu.2019.00423. eCollection 2019. Front Immunol. 2019. PMID: 30915077 Free PMC article.
-
Neutrophil Extracellular Trap Mitochondrial DNA and Its Autoantibody in Systemic Lupus Erythematosus and a Proof-of-Concept Trial of Metformin.Arthritis Rheumatol. 2015 Dec;67(12):3190-200. doi: 10.1002/art.39296. Arthritis Rheumatol. 2015. PMID: 26245802 Clinical Trial.
-
Aberrant expansion of CD177+ neutrophils promotes endothelial dysfunction in systemic lupus erythematosus via neutrophil extracellular traps.J Autoimmun. 2025 Mar;152:103399. doi: 10.1016/j.jaut.2025.103399. Epub 2025 Mar 14. J Autoimmun. 2025. PMID: 40088615
-
Neutrophils-Important Communicators in Systemic Lupus Erythematosus and Antiphospholipid Syndrome.Front Immunol. 2019 Nov 22;10:2734. doi: 10.3389/fimmu.2019.02734. eCollection 2019. Front Immunol. 2019. PMID: 31824510 Free PMC article. Review.
Cited by
-
A Differential Protein Study on Bronchoalveolar Lavage Fluid at Different Stages of Silicosis.Comb Chem High Throughput Screen. 2024;27(16):2366-2401. doi: 10.2174/0113862073260760231023055036. Comb Chem High Throughput Screen. 2024. PMID: 38173059
-
Bone sialoprotein stimulates cancer cell adhesion through the RGD motif and the αvβ3 and αvβ5 integrin receptors.Oncol Lett. 2024 Sep 9;28(5):542. doi: 10.3892/ol.2024.14675. eCollection 2024 Nov. Oncol Lett. 2024. PMID: 39310027 Free PMC article.
-
The Dual Role of Platelets in the Cardiovascular Risk of Chronic Inflammation.Front Immunol. 2021 Apr 1;12:625181. doi: 10.3389/fimmu.2021.625181. eCollection 2021. Front Immunol. 2021. PMID: 33868242 Free PMC article. Review.
-
Detection and functional resolution of soluble immune complexes by an FcγR reporter cell panel.EMBO Mol Med. 2022 Jan 11;14(1):e14182. doi: 10.15252/emmm.202114182. Epub 2021 Nov 29. EMBO Mol Med. 2022. PMID: 34842342 Free PMC article.
-
Selenium Nanoparticles Can Influence the Immune Response Due to Interactions with Antibodies and Modulation of the Physiological State of Granulocytes.Pharmaceutics. 2022 Dec 11;14(12):2772. doi: 10.3390/pharmaceutics14122772. Pharmaceutics. 2022. PMID: 36559266 Free PMC article.
References
-
- Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Annals of the rheumatic diseases. 2015;74:1417–1424. doi: 10.1136/annrheumdis-2013-204837. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

