Diverse hydrocarbon biosynthetic enzymes can substitute for olefin synthase in the cyanobacterium Synechococcus sp. PCC 7002
- PMID: 30718738
- PMCID: PMC6361979
- DOI: 10.1038/s41598-018-38124-y
Diverse hydrocarbon biosynthetic enzymes can substitute for olefin synthase in the cyanobacterium Synechococcus sp. PCC 7002
Abstract
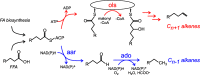
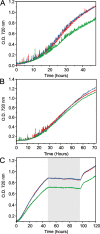
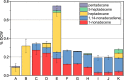
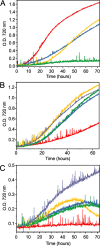
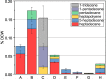
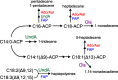
Cyanobacteria are among only a few organisms that naturally synthesize long-chain alkane and alkene hydrocarbons. Cyanobacteria use one of two pathways to synthesize alka/enes, either acyl-ACP reductase (Aar) and aldehyde deformylating oxygenase (Ado) or olefin synthase (Ols). The genomes of cyanobacteria encode one of these pathways but never both, suggesting a mutual exclusivity. We studied hydrocarbon pathway compatibility using the model cyanobacterium Synechococcus sp. PCC 7002 (S7002) by co-expressing Ado/Aar and Ols and by entirely replacing Ols with three other types of hydrocarbon biosynthetic pathways. We find that Ado/Aar and Ols can co-exist and that slower growth occurs only when Ado/Aar are overexpressed at 38 °C. Furthermore, Ado/Aar and the non-cyanobacterial enzymes UndA and fatty acid photodecarboxylase are able to substitute for Ols in a knockout strain and conditionally rescue slow growth. Production of hydrocarbons by UndA in S7002 required a rational mutation to increase substrate range. Expression of the non-native enzymes in S7002 afforded unique hydrocarbon profiles and alka/enes not naturally produced by cyanobacteria. This suggests that the biosynthetic enzyme and the resulting types of hydrocarbons are not critical to supporting growth. Exchanging or mixing hydrocarbon pathways could enable production of novel types of CO2-derived hydrocarbons in cyanobacteria.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Alkane production by the marine cyanobacterium Synechococcus sp. NKBG15041c possessing the α-olefin biosynthesis pathway.Appl Microbiol Biotechnol. 2015 Feb;99(3):1521-9. doi: 10.1007/s00253-014-6286-2. Epub 2014 Dec 21. Appl Microbiol Biotechnol. 2015. PMID: 25527377
-
Electrostatic interactions at the interface of two enzymes are essential for two-step alkane biosynthesis in cyanobacteria.Biosci Biotechnol Biochem. 2020 Feb;84(2):228-237. doi: 10.1080/09168451.2019.1677142. Epub 2019 Oct 10. Biosci Biotechnol Biochem. 2020. PMID: 31601165
-
Characterization of cyanobacterial hydrocarbon composition and distribution of biosynthetic pathways.PLoS One. 2014 Jan 27;9(1):e85140. doi: 10.1371/journal.pone.0085140. eCollection 2014. PLoS One. 2014. PMID: 24475038 Free PMC article.
-
Recent advances in the improvement of cyanobacterial enzymes for bioalkane production.Microb Cell Fact. 2022 Dec 12;21(1):256. doi: 10.1186/s12934-022-01981-4. Microb Cell Fact. 2022. PMID: 36503511 Free PMC article. Review.
-
Cyanobacterial Enzymes for Bioalkane Production.Adv Exp Med Biol. 2018;1080:119-154. doi: 10.1007/978-981-13-0854-3_6. Adv Exp Med Biol. 2018. PMID: 30091094 Review.
Cited by
-
Photosynthetic Conversion of Carbon Dioxide to Oleochemicals by Cyanobacteria: Recent Advances and Future Perspectives.Front Microbiol. 2020 Apr 17;11:634. doi: 10.3389/fmicb.2020.00634. eCollection 2020. Front Microbiol. 2020. PMID: 32362881 Free PMC article. Review.
-
Microbial engineering to produce fatty alcohols and alkanes.J Ind Microbiol Biotechnol. 2021 Apr 30;48(1-2):kuab011. doi: 10.1093/jimb/kuab011. J Ind Microbiol Biotechnol. 2021. PMID: 33713132 Free PMC article. Review.
-
A Novel Fungal Lipase With Methanol Tolerance and Preference for Macaw Palm Oil.Front Bioeng Biotechnol. 2020 May 6;8:304. doi: 10.3389/fbioe.2020.00304. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32435636 Free PMC article.
-
Microbial production and consumption of hydrocarbons in the global ocean.Nat Microbiol. 2021 Apr;6(4):489-498. doi: 10.1038/s41564-020-00859-8. Epub 2021 Feb 1. Nat Microbiol. 2021. PMID: 33526885
-
Insights into cyanobacterial alkane biosynthesis.J Ind Microbiol Biotechnol. 2022 Apr 14;49(2):kuab075. doi: 10.1093/jimb/kuab075. J Ind Microbiol Biotechnol. 2022. PMID: 34718648 Free PMC article. Review.
References
-
- Whitton, B. A. & Potts, M. The ecology of cyanobacteria: their diversity in time and space. (Springer Science & Business Media, 2007).
-
- Han, J., McCarthy, E. D., Calvin, M. & Benn, M. H. Hydrocarbon constituents of the blue-green algae Nostoc muscorum, Anacystis nidulans, Phormidium luridium and Chlorogloea fritschii. Journal of the Chemical Society C: Organic, 2785–2791, 10.1039/J39680002785 (1968).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

