Epithelial to mesenchymal transition is mediated by both TGF-β canonical and non-canonical signaling during axolotl limb regeneration
- PMID: 30718780
- PMCID: PMC6362101
- DOI: 10.1038/s41598-018-38171-5
Epithelial to mesenchymal transition is mediated by both TGF-β canonical and non-canonical signaling during axolotl limb regeneration
Erratum in
-
Author Correction: Epithelial to mesenchymal transition is mediated by both TGF-β canonical and non-canonical signaling during axolotl limb regeneration.Sci Rep. 2023 Jan 17;13(1):926. doi: 10.1038/s41598-023-28207-w. Sci Rep. 2023. PMID: 36650287 Free PMC article. No abstract available.
Abstract
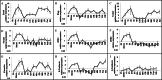
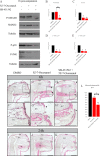
Axolotls have the amazing ability to regenerate. When compared to humans, axolotls display a very fast wound closure, no scarring and are capable to replace lost appendages perfectly. Understanding the signaling mechanism leading to this perfect healing is a key step to help develop regenerative treatments for humans. In this paper, we studied cellular pathways leading to axolotl limb regeneration. We focus on the wound closure phase where keratinocytes migrate to close the lesion site and how epithelial to mesenchymal transitions are involved in this process. We observe a correlation between wound closure and EMT marker expression. Functional analyses using pharmacological inhibitors showed that the TGF-β/SMAD (canonical) and the TGF-β/p38/JNK (non-canonical) pathways play a role in the rate to which the keratinocytes can migrate. When we treat the animals with a combination of inhibitors blocking both canonical and non-canonical TGF-β pathways, it greatly reduced the rate of wound closure and had significant effects on certain known EMT genes.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Putative epithelial-mesenchymal transitions during salamander limb regeneration: Current perspectives and future investigations.Ann N Y Acad Sci. 2024 Oct;1540(1):89-103. doi: 10.1111/nyas.15210. Epub 2024 Sep 13. Ann N Y Acad Sci. 2024. PMID: 39269330 Review.
-
Transforming growth factor: beta signaling is essential for limb regeneration in axolotls.PLoS One. 2007 Nov 28;2(11):e1227. doi: 10.1371/journal.pone.0001227. PLoS One. 2007. PMID: 18043735 Free PMC article.
-
Activation of Smad2 but not Smad3 is required to mediate TGF-β signaling during axolotl limb regeneration.Development. 2016 Oct 1;143(19):3481-3490. doi: 10.1242/dev.131466. Epub 2016 Aug 22. Development. 2016. PMID: 27549395
-
Epithelial-mesenchymal transition, TGF-β, and osteopontin in wound healing and tissue remodeling after injury.J Burn Care Res. 2012 May-Jun;33(3):311-8. doi: 10.1097/BCR.0b013e318240541e. J Burn Care Res. 2012. PMID: 22561306 Free PMC article. Review.
-
SMAD inhibition attenuates epithelial to mesenchymal transition by primary keratinocytes in vitro.Exp Dermatol. 2014 Jul;23(7):497-503. doi: 10.1111/exd.12452. Exp Dermatol. 2014. PMID: 24848428
Cited by
-
Amputation Triggers Long-Range Epidermal Permeability Changes in Evolutionarily Distant Regenerative Organisms.bioRxiv [Preprint]. 2024 Aug 31:2024.08.29.610385. doi: 10.1101/2024.08.29.610385. bioRxiv. 2024. PMID: 39257748 Free PMC article. Preprint.
-
Research Advances in the Mechanisms of Hyperuricemia-Induced Renal Injury.Biomed Res Int. 2020 Jun 26;2020:5817348. doi: 10.1155/2020/5817348. eCollection 2020. Biomed Res Int. 2020. PMID: 32685502 Free PMC article. Review.
-
Putative epithelial-mesenchymal transitions during salamander limb regeneration: Current perspectives and future investigations.Ann N Y Acad Sci. 2024 Oct;1540(1):89-103. doi: 10.1111/nyas.15210. Epub 2024 Sep 13. Ann N Y Acad Sci. 2024. PMID: 39269330 Review.
-
Adrenergic signaling coordinates distant and local responses to amputation in axolotl.bioRxiv [Preprint]. 2025 Jul 24:2021.12.29.474455. doi: 10.1101/2021.12.29.474455. bioRxiv. 2025. PMID: 40777403 Free PMC article. Preprint.
-
Evidence for synergy between sarcomeres and fibroblasts in an in vitro model of myocardial reverse remodeling.J Mol Cell Cardiol. 2021 Sep;158:11-25. doi: 10.1016/j.yjmcc.2021.05.005. Epub 2021 May 14. J Mol Cell Cardiol. 2021. PMID: 33992697 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

