Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts
- PMID: 30718840
- PMCID: PMC6362250
- DOI: 10.1038/s41467-019-08388-7
Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts
Abstract
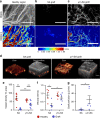
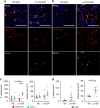
Vascularization and efficient perfusion are long-standing challenges in cardiac tissue engineering. Here we report engineered perfusable microvascular constructs, wherein human embryonic stem cell-derived endothelial cells (hESC-ECs) are seeded both into patterned microchannels and the surrounding collagen matrix. In vitro, the hESC-ECs lining the luminal walls readily sprout and anastomose with de novo-formed endothelial tubes in the matrix under flow. When implanted on infarcted rat hearts, the perfusable microvessel grafts integrate with coronary vasculature to a greater degree than non-perfusable self-assembled constructs at 5 days post-implantation. Optical microangiography imaging reveal that perfusable grafts have 6-fold greater vascular density, 2.5-fold higher vascular velocities and >20-fold higher volumetric perfusion rates. Implantation of perfusable grafts containing additional hESC-derived cardiomyocytes show higher cardiomyocyte and vascular density. Thus, pre-patterned vascular networks enhance vascular remodeling and accelerate coronary perfusion, potentially supporting cardiac tissues after implantation. These findings should facilitate the next generation of cardiac tissue engineering design.
Conflict of interest statement
C.E.M. is a scientific founder and equity holder in Cytocardia. R.K.W. discloses intellectual property (US8180134 In vivo structural and flow imaging (2006), US9013555 Method and apparatus for ultrahigh sensitive optical microangiography (2009)) owned by the Oregon Health and Science University and the University of Washington related to OCT angiography, and licensed to commercial entities, which are related to the technology and analysis methods described in part of this manuscript. R.K.W. also receives research support from Carl Zeiss Meditec Inc, Moptim Inc, Facebook Technologies LLC, and Colgate Palmolive Company. He is a consultant to Insight Photonic Solutions, Kowa, and Carl Zeiss Meditec. All the other authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
- R01HL141570 /NH/NIH HHS/United States
- R01 HL128362/HL/NHLBI NIH HHS/United States
- T32 EB001650/EB/NIBIB NIH HHS/United States
- DP2DK102258 /NH/NIH HHS/United States
- R01HL093140 /NH/NIH HHS/United States
- R01HL128362/NH/NIH HHS/United States
- P01GM081619 /NH/NIH HHS/United States
- P01 GM081619/GM/NIGMS NIH HHS/United States
- R01 HL141570/HL/NHLBI NIH HHS/United States
- P01 HL094374/HL/NHLBI NIH HHS/United States
- P01HL094374/NH/NIH HHS/United States
- DP2 DK102258/DK/NIDDK NIH HHS/United States
- T32HL7312 /NH/NIH HHS/United States
- T32 HL007312/HL/NHLBI NIH HHS/United States
- R01 HL093140/HL/NHLBI NIH HHS/United States
- R01 EY024158/EY/NEI NIH HHS/United States
- R01EY024158 /NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

