Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning
- PMID: 30718903
- PMCID: PMC6410571
- DOI: 10.1038/s41593-018-0334-7
Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning
Abstract
Synapse density is reduced in postmortem cortical tissue from schizophrenia patients, which is suggestive of increased synapse elimination. Using a reprogrammed in vitro model of microglia-mediated synapse engulfment, we demonstrate increased synapse elimination in patient-derived neural cultures and isolated synaptosomes. This excessive synaptic pruning reflects abnormalities in both microglia-like cells and synaptic structures. Further, we find that schizophrenia risk-associated variants within the human complement component 4 locus are associated with increased neuronal complement deposition and synapse uptake; however, they do not fully explain the observed increase in synapse uptake. Finally, we demonstrate that the antibiotic minocycline reduces microglia-mediated synapse uptake in vitro and its use is associated with a modest decrease in incident schizophrenia risk compared to other antibiotics in a cohort of young adults drawn from electronic health records. These findings point to excessive pruning as a potential target for delaying or preventing the onset of schizophrenia in high-risk individuals.
Conflict of interest statement
Competing interests
C.P.G., A.K., K.W., P.B.W., and J.D.B. are employees of Novartis. R.H.P. has served on the scientific advisory boards of Genomind and Psy Therapeutics, and was a consultant to RID Ventures and Takeda (none related to the present work). C.M.S. discloses lecture and consulting fees from Otsuka Pharmaceutical and H. Lundbeck A/S (none related to the present work). None of the other authors declare any competing interests.
Figures
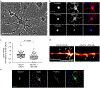

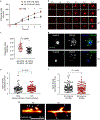
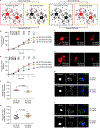
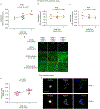
Comment in
-
Microglia, complement and schizophrenia.Nat Neurosci. 2019 Mar;22(3):333-334. doi: 10.1038/s41593-019-0343-1. Nat Neurosci. 2019. PMID: 30796420 No abstract available.
-
Perspectives on Neuroscience and Behavior.Neuroscientist. 2019 Aug;25(4):286-287. doi: 10.1177/1073858419859504. Neuroscientist. 2019. PMID: 31514647 No abstract available.
References
-
- Pantelis C et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 361, 281–288 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

