Adverse Behavioral Changes in Adult Mice Following Neonatal Repeated Exposure to Pain and Sucrose
- PMID: 30719013
- PMCID: PMC6348336
- DOI: 10.3389/fpsyg.2018.02394
Adverse Behavioral Changes in Adult Mice Following Neonatal Repeated Exposure to Pain and Sucrose
Abstract
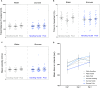
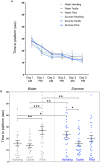
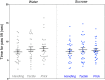
Sucrose is recommended for the treatment of pain during minor procedures in preterm infants in the neonatal intensive care unit (NICU) and is currently used worldwide as the standard of care. We recently reported that adult mice repetitively exposed to sucrose compared to water during the first week of life, irrespective of exposure to an intervention, had significantly smaller brain volumes in large white matter, cortical and subcortical structures (e.g., hippocampus, striatum, fimbria). These structures are important for stress regulation and memory formation. Here, we report the effects of repeated neonatal exposure to pain and sucrose on adult behavior in mice. Neonatal C57BL/6J mice (N = 160, 47% male) were randomly assigned to one of two treatments (sucrose, water) and one of three interventions (needle-prick, tactile, handling). Pups received 10 interventions daily from postnatal day 1 (P1) to P6. A single dose of 24% sucrose or water was given orally 2 min before each intervention. At adulthood (P60-85) mice underwent behavioral testing to assess spatial memory, anxiety, motor function, pain sensitivity, and sugar preference. We found that mice that had received sucrose and handling only, had poorer short-term memory in adulthood compared to water/handling controls (p < 0.05). When exposed to pain, mice treated with repetitive sucrose or water did not differ on memory performance (p = 0.1). A sugar preference test showed that adult mice that received sucrose before an intervention as pups consumed less sugar solution compared to controls or those that received water before pain (p < 0.05). There were no significant group differences in anxiety, motor, or pain sensitivity. In a mouse model that closely mimics NICU care, we show for the first time that memory in adulthood was poorer for mice exposed to pain during the first week of life, irrespective of sucrose treatment, suggesting that sucrose does not protect memory performance when administered for pain. In the absence of pain, early repetitive sucrose exposure induced poorer short-term memory, highlighting the importance of accurate pain assessment.
Keywords: NICU; mouse model; neurodevelopmental outcomes; pain; prematurity; sucrose.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

