Immunotherapy in myeloma: how far have we come?
- PMID: 30719268
- PMCID: PMC6348514
- DOI: 10.1177/2040620718822660
Immunotherapy in myeloma: how far have we come?
Abstract
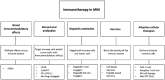
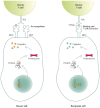
The treatment of multiple myeloma (MM) has evolved substantially over the past decades, leading to a significantly improved outcome of MM patients. The introduction of high-dose therapy, especially, and autologous stem cell transplantation, as well as the development of new drugs, such as immunomodulatory drugs (IMiDs) and proteasome inhibitors have contributed to the improvement in survival. However, eventually most MM patients relapse, which indicates that there is a need for new agents and novel treatment strategies. Importantly, the long-term survival in a subset of MM patients after allogeneic stem cell transplantation illustrates the potential of immunotherapy in MM, but allogeneic stem cell transplantation is also associated with a high rate of treatment-related mortality. Recently, a better insight into several immune-evasion mechanisms, which contribute to tumor progression, has resulted in the development of active and well-tolerated novel forms of immunotherapy. These immunotherapeutic agents can be used as monotherapy, or, even more successfully, in combination with other established anti-MM agents to further improve depth and duration of response by preventing the outgrowth of resistant clones. This review will discuss the mechanisms used by MM cells to evade the immune system, and also provide an overview of currently approved immunotherapeutic drugs, such as IMiDs (e.g. lenalidomide and pomalidomide) and monoclonal antibodies that target cell surface antigens present on the MM cell (e.g. elotuzumab and daratumumab), as well as novel immunotherapies (e.g. chimeric antigen receptor T-cells, bispecific antibodies and checkpoint inhibitors) currently in clinical development in MM.
Keywords: CAR-T cells; IMiDs; bispecific antibodies; checkpoint inhibitors; immunotherapy; monoclonal antibodies; multiple myeloma.
Conflict of interest statement
Conflict of interest statement: NvdD: research support from Celgene, Janssen Pharmaceuticals, Novartis, BMS, and AMGEN; advisory boards for Celgene, Janssen Pharmaceuticals, Novartis, BMS, AMGEN, Takeda, Bayer, and Servier. HML: Research support and honoraria: Celgene and Janssen Pharmaceuticals. The other authors declare no conflicts of interest regarding this work.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

