Clinical and laboratory-induced colistin-resistance mechanisms in Acinetobacter baumannii
- PMID: 30720421
- PMCID: PMC6421349
- DOI: 10.1099/mgen.0.000246
Clinical and laboratory-induced colistin-resistance mechanisms in Acinetobacter baumannii
Abstract
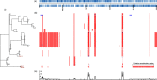
The increasing incidence and emergence of multi-drug resistant (MDR) Acinetobacter baumannii has become a major global health concern. Colistin is a historic antimicrobial that has become commonly used as a treatment for MDR A. baumannii infections. The increase in colistin usage has been mirrored by an increase in colistin resistance. We aimed to identify the mechanisms associated with colistin resistance in A. baumannii using multiple high-throughput-sequencing technologies, including transposon-directed insertion site sequencing (TraDIS), RNA sequencing (RNAseq) and whole-genome sequencing (WGS) to investigate the genotypic changes of colistin resistance in A. baumannii. Using TraDIS, we found that genes involved in drug efflux (adeIJK), and phospholipid (mlaC, mlaF and mlaD) and lipooligosaccharide synthesis (lpxC and lpsO) were required for survival in sub-inhibitory concentrations of colistin. Transcriptomic (RNAseq) analysis revealed that expression of genes encoding efflux proteins (adeI, adeC, emrB, mexB and macAB) was enhanced in in vitro generated colistin-resistant strains. WGS of these organisms identified disruptions in genes involved in lipid A (lpxC) and phospholipid synthesis (mlaA), and in the baeS/R two-component system (TCS). We additionally found that mutations in the pmrB TCS genes were the primary colistin-resistance-associated mechanisms in three Vietnamese clinical colistin-resistant A. baumannii strains. Our results outline the entire range of mechanisms employed in A. baumannii for resistance against colistin, including drug extrusion and the loss of lipid A moieties by gene disruption or modification.
Keywords: Acinetobacter baumannii; RNAseq; TraDIS; colistin resistance; multi-drug resistance; whole-genome sequencing.
Conflict of interest statement
The author(s) declare that there are no conflicts of interest.
Figures

References
-
- Garnacho-Montero J, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar AE, García-Garmendia JL, et al. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis. 2003;36:1111–1118. doi: 10.1086/374337. - DOI - PubMed
-
- Petrosillo N, Chinello P, Proietti MF, Cecchini L, Masala M, et al. Combined colistin and rifampicin therapy for carbapenem-resistant Acinetobacter baumannii infections: clinical outcome and adverse events. Clin Microbiol Infect. 2005;11:682–683. doi: 10.1111/j.1469-0691.2005.01198.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

