β Cell tone is defined by proglucagon peptides through cAMP signaling
- PMID: 30720465
- PMCID: PMC6483521
- DOI: 10.1172/jci.insight.126742
β Cell tone is defined by proglucagon peptides through cAMP signaling
Abstract
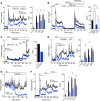
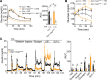
Paracrine interactions between pancreatic islet cells have been proposed as a mechanism to regulate hormone secretion and glucose homeostasis. Here, we demonstrate the importance of proglucagon-derived peptides (PGDPs) for α to β cell communication and control of insulin secretion. Signaling through this system occurs through both the glucagon-like peptide receptor (Glp1r) and glucagon receptor (Gcgr). Loss of PGDPs, or blockade of their receptors, decreases insulin secretion in response to both metabolic and nonmetabolic stimulation of mouse and human islets. This effect is due to reduced β cell cAMP and affects the quantity but not dynamics of insulin release, indicating that PGDPs dictate the magnitude of insulin output in an isolated islet. In healthy mice, additional factors that stimulate cAMP can compensate for loss of PGDP signaling; however, input from α cells is essential to maintain glucose tolerance during the metabolic stress induced by high-fat feeding. These findings demonstrate an essential role for α cell regulation of β cells, raising the possibility that abnormal paracrine signaling contributes to impaired insulin secretion in diabetes. Moreover, these findings support reconsideration of the role for α cells in postprandial glucose control.
Keywords: Endocrinology; G-protein coupled receptors; Insulin; Islet cells; Metabolism.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

