Allergic Reactions Captured by Voluntary Reporting
- PMID: 30720546
- PMCID: PMC6669104
- DOI: 10.1097/PTS.0000000000000568
Allergic Reactions Captured by Voluntary Reporting
Abstract
Background: The epidemiology of hospital adverse reactions (ARs), particularly allergic reactions, or hypersensitivity reactions (HSRs), is poorly defined. To determine priorities for allergy safety in healthcare, we identified and described safety reports of allergic reactions.
Methods: We searched the safety report database of a large academic medical center from April 2006 to March 2016 using 101 complete, truncated, and/or misspelled key words related to allergic symptoms, treatments, and culprits (e.g., medications, foods). Patient and event data were summarized for ARs and two types of ARs, HSRs and side effects/toxicities.
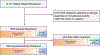
Results: Among 9111 key word search-identified events, 876 (10%) were ARs, of which 436 (5%) were HSRs and the remaining 440 (5%) were side effect reactions or toxicities. Whereas the most common HSRs were simple cutaneous reactions (83%), the following severe immediate HSRs were also identified: shortness of breath (16%), anaphylaxis (14%), and angioedema (12%). Most HSRs were caused by drugs (81%), with antibiotics (26%), particularly β-lactams (11%), and vancomycin (8%), commonly implicated. Other causes of drug HSRs included contrast agents (24%), chemotherapeutics (7%), and opioids (6%). Nondrug HSRs were from blood products (8%), latex (3%), and devices (3%). Food reactions were rarely identified (1%).
Conclusions: We identified ARs, HSRs, and side effects/toxicities, contained in a decade of safety reports at an academic medical center. Allergy safety in the healthcare setting should target approaches to common and severe reactions, with a focus on the safe administration of β-lactams, vancomycin, contrast agents, chemotherapeutics, and opioids. Priority nondrug HSR culprits include blood products, latex, and devices.
Copyright © 2019 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors disclose no conflict of interest.
Figures


References
-
- Institute of Medicine. To Err is Human: Building a Safer Health System Washington, DC: National Academy Press; 2000.
-
- Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy 2016;71(9):1305–13. - PubMed
-
- CRICO. Medication-related malpractice risks. CRICO 2016 CBS Benchmarking Report Available at: https://www.rmf.harvard.edu/Malpractice-Data/Annual-Benchmark-Reports/Ri.... Accessed July 10, 2018.
-
- Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995;274(1):29–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

