Diabetes Impairs Angiogenesis and Induces Endothelial Cell Senescence by Up-Regulating Thrombospondin-CD47-Dependent Signaling
- PMID: 30720765
- PMCID: PMC6386981
- DOI: 10.3390/ijms20030673
Diabetes Impairs Angiogenesis and Induces Endothelial Cell Senescence by Up-Regulating Thrombospondin-CD47-Dependent Signaling
Abstract
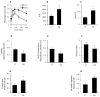
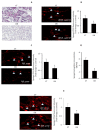
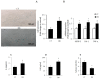
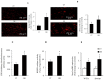
Endothelial dysfunction, impaired angiogenesis and cellular senescence in type 2 diabetes constitute dominant risk factors for chronic non-healing wounds and other cardiovascular disorders. Studying these phenomena in the context of diabetes and the TSP1-CD-47 signaling dictated the use of the in vitro wound endothelial cultured system and an in vivo PVA sponge model of angiogenesis. Herein we report that diabetes impaired the in vivo sponge angiogenic capacity by decreasing cell proliferation, fibrovascular invasion and capillary density. In contrast, a heightened state of oxidative stress and elevated expression of TSP1 and CD47 both at the mRNA and protein levels were evident in this diabetic sponge model of wound healing. An in vitro culturing system involving wound endothelial cells confirmed the increase in ROS generation and the up-regulation of TSP1-CD47 signaling as a function of diabetes. We also provided evidence that diabetic wound endothelial cells (W-ECs) exhibited a characteristic feature that is consistent with cellular senescence. Indeed, enhanced SA-β-gal activity, cell cycle arrest, increased cell cycle inhibitors (CKIs) p53, p21 and p16 and decreased cell cycle promoters including Cyclin D1 and CDK4/6 were all demonstrated in these cells. The functional consequence of this cascade of events was illustrated by a marked reduction in diabetic endothelial cell proliferation, migration and tube formation. A genetic-based strategy in diabetic W-ECs using CD47 siRNA significantly ameliorated in these cells the excessiveness in oxidative stress, attenuation in angiogenic potential and more importantly the inhibition in cell cycle progression and its companion cellular senescence. To this end, the current data provide evidence linking the overexpression of TSP1-CD47 signaling in diabetes to a number of parameters associated with endothelial dysfunction including impaired angiogenesis, cellular senescence and a heightened state of oxidative stress. Moreover, it may also point to TSP1-CD47 as a potential therapeutic target in the treatment of the aforementioned pathologies.
Keywords: cellular senescence TSP1-CD47; diabetes; endothelial dysfunction; impaired angiogenesis; oxidative stress..
Conflict of interest statement
The author declares that they have no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

