Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing
- PMID: 30720911
- PMCID: PMC6850358
- DOI: 10.1002/stem.2988
Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing
Abstract
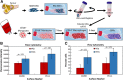
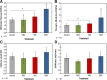
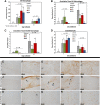
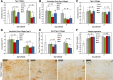
Tendon healing follows a complex series of coordinated events, which ultimately produces a mechanically inferior tissue more scar-like than native tendon. More regenerative healing occurs when anti-inflammatory M2 macrophages play a more dominant role. Mesenchymal stromal/stem cells (MSCs) are able to polarize macrophages to an M2 immunophenotype via paracrine mechanisms. We previously reported that coculture of CD14+ macrophages (MQs) with MSCs resulted in a unique M2-like macrophage. More recently, we generated M2-like macrophages using only extracellular vesicles (EVs) isolated from MSCs creating "EV-educated macrophages" (also called exosome-educated macrophages [EEMs]), thereby foregoing direct use of MSCs. For the current study, we hypothesized that cell therapy with EEMs would improve in vivo tendon healing by modulating tissue inflammation and endogenous macrophage immunophenotypes. We evaluated effects of EEMs using a mouse Achilles tendon rupture model and compared results to normal tendon healing (without any biologic intervention), MSCs, MQs, or EVs. We found that exogenous administration of EEMs directly into the wound promoted a healing response that was significantly more functional and more regenerative. Injured tendons treated with exogenous EEMs exhibited (a) improved mechanical properties, (b) reduced inflammation, and (c) earlier angiogenesis. Treatment with MSC-derived EVs alone were less effective functionally but stimulated a biological response as evidenced by an increased number of endothelial cells and decreased M1/M2 ratio. Because of their regenerative and immunomodulatory effects, EEM treament could provide a novel strategy to promote wound healing in this and various other musculoskeletal injuries or pathologies where inflammation and inadequate healing is problematic. Stem Cells 2019;37:652-662.
Keywords: Achilles tendon injury; Extracellular vesicles; Macrophages; Mesenchymal stromal cells; Tendon healing.
© 2019 The Authors. Stem Cells published by Wiley Periodicals, Inc. on behalf of AlphaMed Press 2019.
Conflict of interest statement
The authors report no potential conflicts of interest.
Figures
References
-
- Mantovani A, Biswas SK, Galdiero MR et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013;229:176–185. - PubMed
-
- Murray PJ. Macrophage polarization. Annu Rev Physiol 2017;79:541–566. - PubMed
-
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: An immunologic functional perspective. Annu Rev Immunol 2009;27:451–483. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

