Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma
- PMID: 30721155
- PMCID: PMC6483505
- DOI: 10.1172/jci.insight.124989
Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma
Abstract
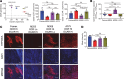
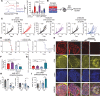
The tumor microenvironment presents physical, immunologic, and metabolic barriers to durable immunotherapy responses. We have recently described roles for both T cell metabolic insufficiency as well as tumor hypoxia as inhibitory mechanisms that prevent T cell activity in murine tumors, but whether intratumoral T cell activity or response to immunotherapy varies between patients as a function of distinct metabolic profiles in tumor cells remains unclear. Here, we show that metabolic derangement can vary widely in both degree and type in patient-derived cell lines and in ex vivo analysis of patient samples, such that some cells demonstrate solely deregulated oxidative or glycolytic metabolism. Further, deregulated oxidative, but not glycolytic, metabolism was associated with increased generation of hypoxia upon implantation into immunodeficient animals. Generation of murine single-cell melanoma cell lines that lacked either oxidative or glycolytic metabolism showed that elevated tumor oxygen consumption was associated with increased T cell exhaustion and decreased immune activity. Moreover, melanoma lines lacking oxidative metabolism were solely responsive to anti-PD-1 therapy among those tested. Prospective analysis of patient sample immunotherapy revealed that oxidative, but not glycolytic, metabolism was associated with progression on PD-1 blockade. Our data highlight a role for oxygen as a crucial metabolite required for the tumor-infiltrating T cells to differentiate appropriately upon PD-1 blockade, and suggest that tumor oxidative metabolism may be a target to improve immunotherapeutic response.
Keywords: Cancer immunotherapy; Glucose metabolism; Immunology; T cells.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

