An SFTPC BRICHOS mutant links epithelial ER stress and spontaneous lung fibrosis
- PMID: 30721158
- PMCID: PMC6483196
- DOI: 10.1172/jci.insight.126125
An SFTPC BRICHOS mutant links epithelial ER stress and spontaneous lung fibrosis
Abstract
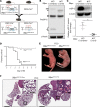
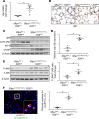
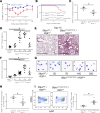
Alveolar type 2 (AT2) cell endoplasmic reticulum (ER) stress is a prominent feature in adult and pediatric interstitial lung disease (ILD and ChILD), but in vivo models linking AT2 cell ER stress to ILD have been elusive. Based on a clinical ChILD case, we identified a critical cysteine residue in the surfactant protein C gene (SFTPC) BRICHOS domain whose mutation induced ER stress in vitro. To model this in vivo, we generated a knockin mouse model expressing a cysteine-to-glycine substitution at codon 121 (C121G) in the Sftpc gene. SftpcC121G expression during fetal development resulted in a toxic gain-of-function causing fatal postnatal respiratory failure from disrupted lung morphogenesis. Induced SftpcC121G expression in adult mice resulted in an ER-retained pro-protein causing AT2 cell ER stress. SftpcC121G AT2 cells were a source of cytokines expressed in concert with development of polycellular alveolitis. These cytokines were subsequently found in a high-dimensional proteomic screen of bronchoalveolar lavage fluid from ChILD patients with the same class of SFTPC mutations. Following alveolitis resolution, SftpcC121G mice developed spontaneous pulmonary fibrosis and restrictive lung impairment. This model provides proof of concept linking AT2 cell ER stress to fibrotic lung disease coupled with translationally relevant biomarkers.
Keywords: Cell stress; Fibrosis; Protein misfolding; Pulmonology.
Conflict of interest statement
Figures



References
-
- Travis WD, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

