Quantitation of all Four Gardnerella vaginalis Clades Detects Abnormal Vaginal Microbiota Characteristic of Bacterial Vaginosis More Accurately than Putative G. vaginalis Sialidase A Gene Count
- PMID: 30721449
- PMCID: PMC6394432
- DOI: 10.1007/s40291-019-00382-5
Quantitation of all Four Gardnerella vaginalis Clades Detects Abnormal Vaginal Microbiota Characteristic of Bacterial Vaginosis More Accurately than Putative G. vaginalis Sialidase A Gene Count
Abstract
Background: Bacterial vaginosis (BV) is a vaginal disorder characterized by a depletion of the normal lactobacillus-dominant microbiota and overgrowth of mainly anaerobic bacteria.
Objectives: The study aimed to evaluate the distribution and abundance of the Gardnerella vaginalis clades and sialidase A gene in vaginal samples from Russian women, and investigate if the G. vaginalis sialidase A gene count detects an abnormal vaginal microbiota characteristic of BV more accurately than G. vaginalis load.
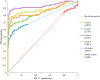
Methods: Vaginal samples from 299 non-pregnant patients of gynecological clinics were examined using Nugent scores and G. vaginalis clade and sialidase A gene quantitative real-time polymerase chain reactions (PCRs). Discriminatory power for BV microbiota was evaluated with receiver operating characteristic (ROC) analysis.
Results: The vaginal microbiota was characterized by Nugent scores as normal, intermediate, and BV microbiota in 162, 58, and 79 women, respectively. G. vaginalis clades 1, 2, 3, 4, and the sialidase A gene were detected in 56% (51-62%), 40% (34-45%), 20% (16-25%), 94% (91-96%), and 70% (64-75%) of vaginal samples, respectively. The frequency and abundance of clades 1, 2, 4, and the sialidase A gene as well as clade multiplicity were significantly associated with abnormal microbiota. The sialidase A gene was present in all multi-clade samples, in all single-clade samples comprising clades 1, 2, and 3, and in four of 84 (5% [2-12%]) samples comprising clade 4 only. Total G. vaginalis load showed significantly higher discriminatory power for abnormal microbiota than sialidase A gene count (areas under ROC curves 0.933 vs. 0.881; p = 0.0306).
Conclusions: Quantifying all four G. vaginalis clades discriminates between BV microbiota and normal microbiota more accurately than measuring G. vaginalis sialidase A gene. Clade 4 is strongly associated with BV microbiota, despite most clade 4 strains lacking the sialidase A gene.
Conflict of interest statement
Conflict of interest
Elena Shipitsyna, Anna Krysanova, Guzel Khayrullina, Kira Shalepo, Alevtina Savicheva, Alexander Guschin, and Magnus Unemo declare that they have no conflicts of interest that are directly relevant to the content of this study.
Ethical approval
The study was approved by the Ethical Committee at the D.O. Ott Research Institute of Obstetrics, Gynaecology and Reproductology, St Petersburg (approval number 73/2015).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures

References
-
- Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–869. doi: 10.1097/OLQ.0b013e318074e565. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

