Slc20a2, Encoding the Phosphate Transporter PiT2, Is an Important Genetic Determinant of Bone Quality and Strength
- PMID: 30721528
- PMCID: PMC6618161
- DOI: 10.1002/jbmr.3691
Slc20a2, Encoding the Phosphate Transporter PiT2, Is an Important Genetic Determinant of Bone Quality and Strength
Abstract
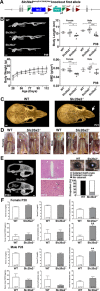
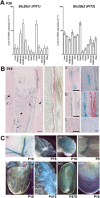
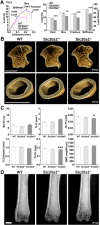
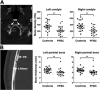
Osteoporosis is characterized by low bone mineral density (BMD) and fragility fracture and affects over 200 million people worldwide. Bone quality describes the material properties that contribute to strength independently of BMD, and its quantitative analysis is a major priority in osteoporosis research. Tissue mineralization is a fundamental process requiring calcium and phosphate transporters. Here we identify impaired bone quality and strength in Slc20a2-/- mice lacking the phosphate transporter SLC20A2. Juveniles had abnormal endochondral and intramembranous ossification, decreased mineral accrual, and short stature. Adults exhibited only small reductions in bone mass and mineralization but a profound impairment of bone strength. Bone quality was severely impaired in Slc20a2-/- mice: yield load (-2.3 SD), maximum load (-1.7 SD), and stiffness (-2.7 SD) were all below values predicted from their bone mineral content as determined in a cohort of 320 wild-type controls. These studies identify Slc20a2 as a physiological regulator of tissue mineralization and highlight its critical role in the determination of bone quality and strength. © 2019 The Authors. Journal of Bone and Mineral Research Published by Wiley Periodicals Inc.
Keywords: ANIMAL MODELS (GENETIC ANIMAL MODELS); BONE MATRIX (MATRIX MINERALIZATION); DISORDERS OF CALCIUM/PHOSPHATE METABOLISM (OTHER); GENETIC RESEARCH (HUMAN ASSOCIATION STUDIES); ORTHOPAEDICS (BIOMECHANICS).
© 2019 The Authors. Journal of Bone and Mineral Research Published by Wiley Periodicals Inc.
Figures




References
-
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33. - PubMed
-
- Compston J. Bone quality: what is it and how is it measured? Arq Bras Endocrinol Metabol. 2006;50(4):579–85. - PubMed
-
- Anderson HC, Garimella R, Tague SE. The role of matrix vesicles in growth plate development and biomineralization. Front Biosci. 2005;10:822–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

